TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation - PubMed (original) (raw)
TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation
Andrea Di Pietro et al. J Virol. 2013 Apr.
Abstract
Tripartite motif (TRIM) protein superfamily members are emerging as important effectors of the innate immune response against viral infections. In particular, TRIM22 was reported to exert antiviral activity against RNA viruses, such as hepatitis B virus (HBV), encephalomyocarditis virus (ECMV), and human immunodeficiency virus type 1 (HIV-1). We demonstrate here, for the first time, that TRIM22 is upregulated by influenza A virus (IAV) infection at both mRNA and protein levels in human alveolar epithelial A549 cells. Conversely, TRIM22 potently restricted IAV replication, in that prevention of TRIM22 expression by means of short hairpin RNA led to a 10-fold enhancement of IAV replication in these cells. Depletion of TRIM22 also reduced the anti-IAV activity of alpha interferon (IFN-α), suggesting that TRIM22 is an important IFN-stimulated gene that is required for maximal suppression of IAV by type I IFN. Furthermore, the IAV infectious titer decreased up to 100-fold in MDCK cells expressing exogenous human TRIM22. Restriction of IAV replication was accounted for by the interaction between TRIM22 and the viral nucleoprotein (NP), resulting in its polyubiquitination and degradation in a proteasome-dependent manner. Thus, TRIM22 represents a novel restriction factor upregulated upon IAV infection that curtails its replicative capacity in epithelial cells.
Figures
Fig 1
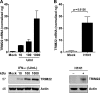
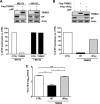
IFN-α exposure and IAV infection induce TRIM22 expression in A549 cells. (A) A549 cells were exposed to increasing doses of IFN-α for 20 h. (B) A549 cells were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 5. TRIM22 mRNA levels (upper) and protein expression (lower) were evaluated 24 h postexposure and were compared to those of mock-treated cells.
Fig 2
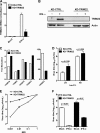
Endogenous TRIM22 contributes to controlling IAV replication in A549 cells. (A) Stable knockdown of endogenous TRIM22 (KD-TRIM22) was obtained in A549 cells through lentiviral transduction, and levels of TRIM22 mRNA were evaluated in the presence or absence of IFN-α stimulation (100 U/ml for 20 h). (B) Protein expression levels in KD-TRIM22 A549 cells were undetectable by Western blotting upon stimulation with 100 U/ml of IFN-α for 20 h. Cells transduced with a lentiviral vector expressing a nonsilencing shRNA (KD-CTRL) were used as a control. (C) Real-time PCR of TRIM family members was performed on total cDNA from either KD-CTRL or KD-TRIM22 A549 cells. The fold modulation of the target transcript in cells stimulated with IFN-α (100 U/ml) was related to that of untreated control cells. Of note, TRIM22 was significantly more expressed than the other TRIMs upon IFN-α stimulation. One experiment of three performed is shown. (D) KD-CTRL and KD-TRIM22 cells were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 0.01. Viral replication was measured by titrating infectious particle production in culture supernatants 24 and 48 h p.i. (E) KD-CTRL and KD-TRIM22 cells were infected with A/NewCaledonia/20/99 (H1N1) at an increasing MOI. Viral replication was measured by titrating infectious particle production in culture supernatants 24 h p.i. (F) Viral titers were determined in the supernatant from KD-CTRL and KD-TRIM22 A549 cells that were either mock treated or treated with IFN-α at 100 U/ml 24 h prior to IAV infection at an MOI of 0.01. Twenty-four h p.i., culture supernatants were collected and virus titers were determined by plaque assay in MDCK cells. All results are the means ± SD of experiments performed in triplicate, and the Western blots are representative of one of three independently performed. P values were determined with paired t test.
Fig 3
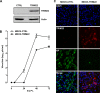
Overexpression of TRIM22 significantly impairs IAV replication in MDCK cells. (A) MDCK cells were stably transduced with a retroviral vector expressing HA-tagged TRIM22 (MDCK-TRIM22) or an empty vector control (MDCK-CTRL). TRIM22 expression was evaluated by Western blotting in whole-cell extracts using an anti-HA Ab. Actin was detected as a control input. (B) Stably transduced MDCK cells were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 0.001, and the kinetics of viral replication were measured by titrating infectious particles produced at the time points shown. Viral titers are expressed as the means ± SD of one representative of three experiments performed in triplicate. (C) To assess the impact of TRIM22 during a single round of IAV replication, stably transduced MDCK cells were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 2. Cells were fixed and stained for TRIM22 (anti-HA) and viral NP at 6 h p.i. Images were acquired by confocal microscopy. The immunofluorescence images are representative of one of three independent experiments.
Fig 4
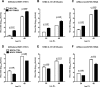
TRIM22 is active against major human-transmissible IAV strains. (A) A549 cells depleted for TRIM22 (KD-TRIM22) or control cells (KD-CTRL) were infected with A/Brisbane/59/07 (H1N1), (B) NYMC X-181 (H1N1pdm), and (C) A/Wisconsin/67/05 at an MOI of 0.01. Viral replication was measured by titrating infectious particles produced at 24 and 48 h p.i. (D, E, and F). Stably transduced MDCK cells were infected with the same virus strains at an MOI of 0.001. The production of infectious virus was measured by titrating infectious particles produced at 24 and 48 h. Viral titers are expressed as the means ± SD of one representative of two experiments performed in triplicate.
Fig 5
TRIM22 leads to impaired NP expression through protein-protein interaction. (A) Control and TRIM22-transduced MDCK cells (CTRL and TRIM22, respectively) were infected with A/NewCaledonia/20/99 (H1N1) at an MOI of 0.001, and the levels of viral NP expression were evaluated in WCE obtained 24 h p.i. Actin was used as a normalizer. (B) 293T cells were cotransfected with a fixed amount of both viral NP-expressing plasmid (50 ng) and GFP-expressing plasmid (50 ng) with increasing amounts of TRIM22-expressing plasmid (from 50 to 5,000 ng). WCE were prepared 24 h p.t., and TRIM22, NP, and GFP expression levels were evaluated by Western blotting. Actin was detected as a loading control. These results are representative of three independent experiments. (C) 293T cells were cotransfected with equal amounts (7.5 μg) of an NP-expressing and a Flag-TRIM22-expressing plasmid or a U1A-expressing plasmid as a control. WCE were prepared 48 h p.t., and immunoprecipitation (IP) with an anti-NP Ab was performed. Input (left) and pulldown product (right) were analyzed by Western blotting for the presence of NP, U1A, and TRIM22. α-Tubulin was detected as a loading control on input extracts.
Fig 6
TRIM22 inhibits IAV replication by degrading the viral nucleoprotein. (A) 293T cells were cotransfected with a fixed amount (50 ng) of NP-expressing plasmid and a 50-fold excess of either TRIM22-expressing or empty vector in the presence or absence of MG132 (2 μM). WCE were prepared 24 h p.t. and analyzed for TRIM22 and NP expression by Western blotting. Actin was used as a normalizer, and relative quantification of detected signal was performed using Image J software (lower panel). (B) 293T cells were transfected with a fixed amount (50 ng) of NP-expressing plasmid and a 50-fold excess of either wild-type (WT) TRIM22 or mutant TRIM22 lacking the N-terminal RING domain (ΔRING). WCE were prepared 24 h p.t., and expression of TRIM22 (anti-FLAG) and NP (anti-NP) was analyzed by Western blotting. Actin was used as a normalizer. Relative quantification of signal was performed using Image J software (lower panel). (C) MDCK cells were transfected with WT or ΔRING mutant TRIM22-expressing plasmid followed by infection with A/NewCaledonia/20/99 (H1N1) at an MOI of 0.001. Infectious particle production was evaluated from culture supernatants collected at 48 h p.i. Results are expressed as the means ± SD of one representative out of three experiments performed in triplicate. P values were determined using one-way analysis of variance. Western blots shown in A and B are representative of 2 independent experiments.
Fig 7
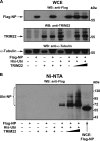
TRIM22 induces ubiquitination of NP. (A) Flag-NP was coexpressed with His-Ubi and increasing amounts of TRIM22 in 293T cells. Forty-eight h after transfection, the cells were treated with 10 μM MG132 for 3 h. Subsequently, WCE were prepared from a 10% fraction of the cells and analyzed by Western blotting using anti-Flag (upper), anti-TRIM22 (middle), and anti-tubulin (lower) antibodies. (B) The remaining 90% of the cells were lysed in denaturing conditions, and ubiquitinated proteins were precipitated using Ni-NTA agarose. Purified ubiquitinated proteins were analyzed by Western blotting for the presence of ubiquitinated forms of Flag-NP (Ubi-NP) using anti-Flag antibodies. WCE from Flag-NP-expressing 293T cells (WCE Flag-NP) was loaded on the gel for size comparison.
Similar articles
- Mutations Conferring Increased Sensitivity to Tripartite Motif 22 Restriction Accumulated Progressively in the Nucleoprotein of Seasonal Influenza A (H1N1) Viruses between 1918 and 2009.
Pagani I, Di Pietro A, Oteiza A, Ghitti M, Mechti N, Naffakh N, Vicenzi E. Pagani I, et al. mSphere. 2018 Apr 4;3(2):e00110-18. doi: 10.1128/mSphere.00110-18. Print 2018 Apr 25. mSphere. 2018. PMID: 29624498 Free PMC article. - TRIM22. A Multitasking Antiviral Factor.
Pagani I, Poli G, Vicenzi E. Pagani I, et al. Cells. 2021 Jul 23;10(8):1864. doi: 10.3390/cells10081864. Cells. 2021. PMID: 34440633 Free PMC article. Review. - MicroRNA-376b-3p Promotes Porcine Reproductive and Respiratory Syndrome Virus Replication by Targeting Viral Restriction Factor TRIM22.
Chen J, Zhao S, Cui Z, Li W, Xu P, Liu H, Miao X, Chen Y, Han F, Zhang H, Xia P, Zhang Y. Chen J, et al. J Virol. 2022 Jan 26;96(2):e0159721. doi: 10.1128/JVI.01597-21. Epub 2021 Nov 10. J Virol. 2022. PMID: 34757838 Free PMC article. - TRIM41-Mediated Ubiquitination of Nucleoprotein Limits Influenza A Virus Infection.
Patil G, Zhao M, Song K, Hao W, Bouchereau D, Wang L, Li S. Patil G, et al. J Virol. 2018 Jul 31;92(16):e00905-18. doi: 10.1128/JVI.00905-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29899090 Free PMC article. - The interferon-stimulated gene TRIM22: A double-edged sword in HIV-1 infection.
Vicenzi E, Poli G. Vicenzi E, et al. Cytokine Growth Factor Rev. 2018 Apr;40:40-47. doi: 10.1016/j.cytogfr.2018.02.001. Epub 2018 Feb 10. Cytokine Growth Factor Rev. 2018. PMID: 29650252 Review.
Cited by
- TRIM Proteins and Their Roles in the Influenza Virus Life Cycle.
Lee HR, Lee MK, Kim CW, Kim M. Lee HR, et al. Microorganisms. 2020 Sep 16;8(9):1424. doi: 10.3390/microorganisms8091424. Microorganisms. 2020. PMID: 32947942 Free PMC article. Review. - Rabies virus uniquely reprograms the transcriptome of human monocyte-derived macrophages.
Embregts CWE, Wentzel AS, den Dekker AT, van IJcken WFJ, Stadhouders R, GeurtsvanKessel CH. Embregts CWE, et al. Front Cell Infect Microbiol. 2023 Jan 31;13:1013842. doi: 10.3389/fcimb.2023.1013842. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36798087 Free PMC article. - Pathogen-driven CRISPR screens identify TREX1 as a regulator of DNA self-sensing during influenza virus infection.
King CR, Liu Y, Amato KA, Schaack GA, Hu T, Smith JA, Mehle A. King CR, et al. bioRxiv [Preprint]. 2023 Feb 7:2023.02.07.527556. doi: 10.1101/2023.02.07.527556. bioRxiv. 2023. PMID: 36798235 Free PMC article. Updated. Preprint. - KAP1 Positively Modulates Influenza A Virus Replication by Interacting with PB2 and NS1 Proteins in Human Lung Epithelial Cells.
Feng H, Yi R, Wu S, Wang G, Sun R, Lin L, Zhu S, Nie Z, He Y, Wang S, Wang P, Shu J, Wu L. Feng H, et al. Viruses. 2022 Mar 26;14(4):689. doi: 10.3390/v14040689. Viruses. 2022. PMID: 35458419 Free PMC article. - An Improved, Dual-Direction, Promoter-Driven, Reverse Genetics System for the Infectious Bursal Disease Virus (IBDV).
Hu X, Chen Z, Wu X, Ding Z, Zeng Q, Wu H. Hu X, et al. Viruses. 2022 Jun 27;14(7):1396. doi: 10.3390/v14071396. Viruses. 2022. PMID: 35891377 Free PMC article.
References
- Capua I, Kajaste-Rudnitski A, Bertoli E, Vicenzi E. 2009. Pandemic vaccine preparedness–have we left something behind? PLoS Pathog. 5:e1000482 doi:10.1371/journal.ppat.1000482 - DOI - PMC - PubMed
- Katze MG, He Y, Gale M., Jr 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675–687 - PubMed
- Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. 2005. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571–5580 - PubMed
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730–737 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G9721629/MRC_/Medical Research Council/United Kingdom
- 090940/WT_/Wellcome Trust/United Kingdom
- G0501446/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- G0900950/MRC_/Medical Research Council/United Kingdom
- G0801172/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous