Bridging the transgenerational gap with epigenetic memory - PubMed (original) (raw)
Review
Bridging the transgenerational gap with epigenetic memory
Jana P Lim et al. Trends Genet. 2013 Mar.
Abstract
It is textbook knowledge that inheritance of traits is governed by genetics, and that the epigenetic modifications an organism acquires are largely reset between generations. Recently, however, transgenerational epigenetic inheritance has emerged as a rapidly growing field, providing evidence suggesting that some epigenetic changes result in persistent phenotypes across generations. Here, we survey some of the most recent examples of transgenerational epigenetic inheritance in animals, ranging from Caenorhabditis elegans to humans, and describe approaches and limitations to studying this phenomenon. We also review the current body of evidence implicating chromatin modifications and RNA molecules in mechanisms underlying this unconventional mode of inheritance and discuss its evolutionary implications.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare no financial conflicts of interest.
Figures
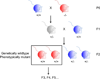
Figure 1. Transgenerational epigenetic inheritance induced by genetic modification in ancestors
A) Crossing scheme used in rodent studies to generate genetically wild type progeny from a mutant ancestor. Color of mouse denotes phenotype (blue are wild type and red are mutant). Note that in many instances presented in the review, the phenotype of the F1 heterozygotes is unknown. B)Crossing scheme used in worm studies to generate genetically wild type progeny from a mutant ancestor. The F1 generation arises from mating between a hermaphrodite and male. The F2 generation arises from the self-fertilization of F1 hermaphrodites. Color of worm denotes phenotype (blue are wild type and red are mutant). Note that in many instances presented here, the phenotype of the F1 heterozygotes is unknown.
Figure 1. Transgenerational epigenetic inheritance induced by genetic modification in ancestors
A) Crossing scheme used in rodent studies to generate genetically wild type progeny from a mutant ancestor. Color of mouse denotes phenotype (blue are wild type and red are mutant). Note that in many instances presented in the review, the phenotype of the F1 heterozygotes is unknown. B)Crossing scheme used in worm studies to generate genetically wild type progeny from a mutant ancestor. The F1 generation arises from mating between a hermaphrodite and male. The F2 generation arises from the self-fertilization of F1 hermaphrodites. Color of worm denotes phenotype (blue are wild type and red are mutant). Note that in many instances presented here, the phenotype of the F1 heterozygotes is unknown.
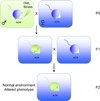
Figure 2. Transgenerational epigenetic inheritance induced by environmental manipulations in ancestors
A) Strategy used to generate progeny from a paternal ancestor subject to an environmental perturbation. This scheme minimizes maternal effects in organisms that require significant maternal care during development. Blue: normal phenotype. Green: altered phenotype resulting from a perturbation to the ancestral environment. B)Scheme used to generate progeny from a maternal ancestor subject to an environmental perturbation. This scheme allows for the study of transgenerational effects induced by an initial change in maternal environment, but minimizes cryptic maternal effects. Blue: normal phenotype. Green: altered phenotype resulting from a perturbation to the ancestral environment.
Figure 2. Transgenerational epigenetic inheritance induced by environmental manipulations in ancestors
A) Strategy used to generate progeny from a paternal ancestor subject to an environmental perturbation. This scheme minimizes maternal effects in organisms that require significant maternal care during development. Blue: normal phenotype. Green: altered phenotype resulting from a perturbation to the ancestral environment. B)Scheme used to generate progeny from a maternal ancestor subject to an environmental perturbation. This scheme allows for the study of transgenerational effects induced by an initial change in maternal environment, but minimizes cryptic maternal effects. Blue: normal phenotype. Green: altered phenotype resulting from a perturbation to the ancestral environment.
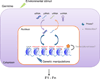
Figure 3. Model of proposed mechanisms of transgenerational epigenetic inheritance
Alterations to the parental genome (aqua arrow) or environmental stimuli (green arrow) could trigger epigenetic changes in the germline of the organism that could be transmitted to the next generation. Such epigenetic changes could possibly be relayed or amplified in the germline of subsequent generations, and persist for several generations. Eventually, epigenetic changes would be reset to a basal state. So far, the epigenetic mechanisms that have been described include changes to chromatin (histone marks, DNA methylation) and changes to non-coding RNAs, in particular those involving the nuclear RNAi pathway. An amplification loop could be initiated by alteration in chromatin marks (e.g. H3K9me3) at a genomic locus, followed by the generation of non-coding RNAs at this particular locus, which would then be transmitted via the germline, and in turn guide H3K9me3 deposition at that same genomic locus in the germline of the next generation. In grey are additional potential mechanisms that remain to be investigated (Box 2).
Similar articles
- Transgenerational Inheritance of Environmentally Induced Epigenetic Alterations during Mammalian Development.
Legoff L, D'Cruz SC, Tevosian S, Primig M, Smagulova F. Legoff L, et al. Cells. 2019 Dec 3;8(12):1559. doi: 10.3390/cells8121559. Cells. 2019. PMID: 31816913 Free PMC article. Review. - Chromatin Modifiers SET-25 and SET-32 Are Required for Establishment but Not Long-Term Maintenance of Transgenerational Epigenetic Inheritance.
Woodhouse RM, Buchmann G, Hoe M, Harney DJ, Low JKK, Larance M, Boag PR, Ashe A. Woodhouse RM, et al. Cell Rep. 2018 Nov 20;25(8):2259-2272.e5. doi: 10.1016/j.celrep.2018.10.085. Cell Rep. 2018. PMID: 30463020 - How do histone modifications contribute to transgenerational epigenetic inheritance in C. elegans?
Woodhouse RM, Ashe A. Woodhouse RM, et al. Biochem Soc Trans. 2020 Jun 30;48(3):1019-1034. doi: 10.1042/BST20190944. Biochem Soc Trans. 2020. PMID: 32539084 Review. - Transgenerational inheritance: how impacts to the epigenetic and genetic information of parents affect offspring health.
Xavier MJ, Roman SD, Aitken RJ, Nixon B. Xavier MJ, et al. Hum Reprod Update. 2019 Sep 11;25(5):518-540. doi: 10.1093/humupd/dmz017. Hum Reprod Update. 2019. PMID: 31374565 - Mechanisms of transgenerational epigenetic inheritance: lessons from animal model organisms.
Santilli F, Boskovic A. Santilli F, et al. Curr Opin Genet Dev. 2023 Apr;79:102024. doi: 10.1016/j.gde.2023.102024. Epub 2023 Mar 7. Curr Opin Genet Dev. 2023. PMID: 36893483 Review.
Cited by
- When is incomplete epigenetic resetting in germ cells favoured by natural selection?
Uller T, English S, Pen I. Uller T, et al. Proc Biol Sci. 2015 Jul 22;282(1811):20150682. doi: 10.1098/rspb.2015.0682. Proc Biol Sci. 2015. PMID: 26136447 Free PMC article. - The information value of non-genetic inheritance in plants and animals.
English S, Pen I, Shea N, Uller T. English S, et al. PLoS One. 2015 Jan 20;10(1):e0116996. doi: 10.1371/journal.pone.0116996. eCollection 2015. PLoS One. 2015. PMID: 25603120 Free PMC article. - Biological embedding of experience: A primer on epigenetics.
Aristizabal MJ, Anreiter I, Halldorsdottir T, Odgers CL, McDade TW, Goldenberg A, Mostafavi S, Kobor MS, Binder EB, Sokolowski MB, O'Donnell KJ. Aristizabal MJ, et al. Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23261-23269. doi: 10.1073/pnas.1820838116. Epub 2019 Oct 17. Proc Natl Acad Sci U S A. 2020. PMID: 31624126 Free PMC article. Review. - Bound Together: How Psychoanalysis Diminishes Inter-generational DNA Trauma.
Colangeli R. Colangeli R. Am J Psychoanal. 2020 Jun;80(2):196-218. doi: 10.1057/s11231-020-09247-x. Am J Psychoanal. 2020. PMID: 32488025 - Epigenetic inheritance and the missing heritability.
Trerotola M, Relli V, Simeone P, Alberti S. Trerotola M, et al. Hum Genomics. 2015 Jul 28;9(1):17. doi: 10.1186/s40246-015-0041-3. Hum Genomics. 2015. PMID: 26216216 Free PMC article. Review.
References
- Kammerer P. The inheritance of acquired characteristics. New York: Boni and Liveright; 1924.
- Vargas AO. Did Paul Kammerer discover epigenetic inheritance? A modern look at the controversial midwife toad experiments. J Exp Zool B Mol Dev Evol. 2009;312:667–678. - PubMed
- Waddington CH. The epigenotype. Endeavor. 1942;1:18–20.
- Waddington CH. Genetic Assimilation of an Acquired Character. Evolution. 1953;7:118–126.
Publication types
MeSH terms
Grants and funding
- T32 MH020016/MH/NIMH NIH HHS/United States
- DP1-AG044848/AG/NIA NIH HHS/United States
- R01-AG031198/AG/NIA NIH HHS/United States
- T32-MH020016/MH/NIMH NIH HHS/United States
- R01 AG031198/AG/NIA NIH HHS/United States
- DP1 AG044848/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous