Extracellular vesicles: exosomes, microvesicles, and friends - PubMed (original) (raw)
Review
Extracellular vesicles: exosomes, microvesicles, and friends
Graça Raposo et al. J Cell Biol. 2013.
Abstract
Cells release into the extracellular environment diverse types of membrane vesicles of endosomal and plasma membrane origin called exosomes and microvesicles, respectively. These extracellular vesicles (EVs) represent an important mode of intercellular communication by serving as vehicles for transfer between cells of membrane and cytosolic proteins, lipids, and RNA. Deficiencies in our knowledge of the molecular mechanisms for EV formation and lack of methods to interfere with the packaging of cargo or with vesicle release, however, still hamper identification of their physiological relevance in vivo. In this review, we focus on the characterization of EVs and on currently proposed mechanisms for their formation, targeting, and function.
Figures
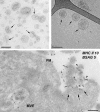
Figure 1.
Ultrastructure of exosomes. (top left) Exosomes isolated from melanoma cells were contrasted with uranyl-acetate and embedded as whole mount preparations in methylcellulose. Note their artificial cup shape appearance (examples are indicated with arrows) and heterogeneous size ranging from 30 to 100 nm. (top right) Exosomes from prostate epithelial cells (prostasomes) were directly frozen and observed by cryo–electron microscopy without chemical fixation or contrasting. Exosomes appear round and are visualized with improved resolution (arrows). The elongated structure (top right of the micrograph) is the Formvar film on the EM grid. (bottom) EBV-transformed B lymphocytes were allowed to endocytose BSA coupled to 5-nm gold particles (BSAG 5) for 10 min and then chased for 20 min in the absence of BSAG 5. Ultrathin cryosections were immunolabeled for MHC class II with 10-nm protein A gold. An MVE fusion profile (arrows) is defined by regurgitated 5-nm BSAG 5 that had previously been endocytosed. In addition to BSAG 5 (arrowheads), the exocytic profile contains exosomes labeled for MHC class II with 10-nm gold (MHC II 10; small arrows). PM, plasma membrane. Bars, 100 nm.
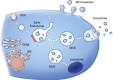
Figure 2.
Release of MVs and exosomes. MVs bud directly from the plasma membrane, whereas exosomes are represented by small vesicles of different sizes that are formed as the ILV by budding into early endosomes and MVEs and are released by fusion of MVEs with the plasma membrane. Other MVEs fuse with lysosomes. The point of divergence between these types of MVEs is drawn at early endosomes, but the existence of distinct early endosomes feeding into these two pathways cannot be excluded. Red spots symbolize clathrin associated with vesicles at the plasma membrane (clathrin-coated vesicles [CCV]) or bilayered clathrin coats at endosomes. Membrane-associated and transmembrane proteins on vesicles are represented as triangles and rectangles, respectively. Arrows represent proposed directions of protein and lipid transport between organelles and between MVEs and the plasma membrane for exosome secretion.
Figure 3.
Schematic of protein and RNA transfer by EVs. Membrane-associated (triangles) and transmembrane proteins (rectangles) and RNAs (curved symbols) are selectively incorporated into the ILV of MVEs or into MVs budding from the plasma membrane. MVEs fuse with the plasma membrane to release exosomes into the extracellular milieu. MVs and exosomes may dock at the plasma membrane of a target cell (1). Bound vesicles may either fuse directly with the plasma membrane (2) or be endocytosed (3). Endocytosed vesicles may then fuse with the delimiting membrane of an endocytic compartment (4). Both pathways result in the delivery of proteins and RNA into the membrane or cytosol of the target cell. Fusion and endocytosis are only represented for exosomal vesicles, but plasma membrane–derived MVs may have similar fates.
Similar articles
- Socially Distanced Intercellular Communication: Mechanisms for Extracellular Vesicle Cargo Delivery.
Popa SJ, Stewart SE. Popa SJ, et al. Subcell Biochem. 2021;97:179-209. doi: 10.1007/978-3-030-67171-6_8. Subcell Biochem. 2021. PMID: 33779918 - Shedding light on the cell biology of extracellular vesicles.
van Niel G, D'Angelo G, Raposo G. van Niel G, et al. Nat Rev Mol Cell Biol. 2018 Apr;19(4):213-228. doi: 10.1038/nrm.2017.125. Epub 2018 Jan 17. Nat Rev Mol Cell Biol. 2018. PMID: 29339798 Review. - Extracellular vesicles shuffling intercellular messages: for good or for bad.
Lo Cicero A, Stahl PD, Raposo G. Lo Cicero A, et al. Curr Opin Cell Biol. 2015 Aug;35:69-77. doi: 10.1016/j.ceb.2015.04.013. Epub 2015 May 19. Curr Opin Cell Biol. 2015. PMID: 26001269 Review. - [Progress in extracellular vesicle imaging methods].
Wang K, Wei Y, Zhang P, Wang J, Hu J, Wang L, Li B. Wang K, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2020 Feb 29;40(2):279-286. doi: 10.12122/j.issn.1673-4254.2020.02.22. Nan Fang Yi Ke Da Xue Xue Bao. 2020. PMID: 32376541 Free PMC article. Review. Chinese. - Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake.
Abels ER, Breakefield XO. Abels ER, et al. Cell Mol Neurobiol. 2016 Apr;36(3):301-12. doi: 10.1007/s10571-016-0366-z. Epub 2016 Apr 6. Cell Mol Neurobiol. 2016. PMID: 27053351 Free PMC article. Review.
Cited by
- Characterization of exosome-mediated propagation of systemic inflammatory responses into the central nervous system.
Kodali MC, Salim C, Ismael S, Lebovitz SG, Lin G, Liao FF. Kodali MC, et al. Mol Brain. 2024 Nov 15;17(1):80. doi: 10.1186/s13041-024-01120-7. Mol Brain. 2024. PMID: 39548504 Free PMC article. - Harmonising cellular conversations: decoding the vital roles of extracellular vesicles in respiratory system intercellular communications.
Jadamba B, Jin Y, Lee H. Jadamba B, et al. Eur Respir Rev. 2024 Nov 13;33(174):230272. doi: 10.1183/16000617.0272-2023. Print 2024 Oct. Eur Respir Rev. 2024. PMID: 39537245 Free PMC article. Review. - Synthetic circRNA therapeutics: innovations, strategies, and future horizons.
Cai J, Qiu Z, Chi-Shing Cho W, Liu Z, Chen S, Li H, Chen K, Li Y, Zuo C, Qiu M. Cai J, et al. MedComm (2020). 2024 Nov 9;5(11):e720. doi: 10.1002/mco2.720. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39525953 Free PMC article. Review. - Updating Research on Extracellular Vesicles of the Male Reproductive Tract in Farm Animals: A Systematic Review.
Martínez-Díaz P, Parra A, Montesdeoca M, Barranco I, Roca J. Martínez-Díaz P, et al. Animals (Basel). 2024 Oct 31;14(21):3135. doi: 10.3390/ani14213135. Animals (Basel). 2024. PMID: 39518859 Free PMC article. Review. - Unlocking the Secrets of Extracellular Vesicles: Orchestrating Tumor Microenvironment Dynamics in Metastasis, Drug Resistance, and Immune Evasion.
Mir R, Baba SK, Elfaki I, Algehainy N, Alanazi MA, Altemani FH, Tayeb FJ, Barnawi J, Husain E, Bedaiwi RI, Albalawi IA, Alhujaily M, Mir MM, Almotairi R, Alatwi HE, Albalawi AD. Mir R, et al. J Cancer. 2024 Oct 14;15(19):6383-6415. doi: 10.7150/jca.98426. eCollection 2024. J Cancer. 2024. PMID: 39513123 Free PMC article. Review.
References
- Aalberts M., van Dissel-Emiliani F.M., van Adrichem N.P., van Wijnen M., Wauben M.H., Stout T.A., Stoorvogel W. 2012. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol. Reprod. 86:82 10.1095/biolreprod.111.095760 - DOI - PubMed
- Admyre C., Johansson S.M., Qazi K.R., Filén J.J., Lahesmaa R., Norman M., Neve E.P., Scheynius A., Gabrielsson S. 2007. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179:1969–1978 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources