Cerebral amyloid angiopathy burden associated with leukoaraiosis: a positron emission tomography/magnetic resonance imaging study - PubMed (original) (raw)
doi: 10.1002/ana.23830. Epub 2013 Feb 19.
Anand Viswanathan, Christopher Gidicsin, Trey Hedden, Sergi Martinez-Ramirez, Andrew Dumas, Anastasia Vashkevich, Alison M Ayres, Eitan Auriel, Ellis van Etten, Alex Becker, Jeremy Carmasin, Kristin Schwab, Jonathan Rosand, Keith A Johnson, Steven M Greenberg
Affiliations
- PMID: 23424091
- PMCID: PMC3715595
- DOI: 10.1002/ana.23830
Cerebral amyloid angiopathy burden associated with leukoaraiosis: a positron emission tomography/magnetic resonance imaging study
M Edip Gurol et al. Ann Neurol. 2013 Apr.
Abstract
Objective: We hypothesized that vascular amyloid contributes to chronic brain ischemia, therefore amyloid burden measured by Pittsburgh compound B retention on positron emission tomography (PiB PET) would correlate with the extent of magnetic resonance imaging (MRI) white matter hyperintensities (WMH; or leukoaraiosis) in patients with high vascular amyloid deposition (cerebral amyloid angiopathy [CAA]) but not in patients with high parenchymal amyloid deposition (Alzheimer disease [AD]; mild cognitive impairment [MCI]) or in healthy elderly (HE) subjects.
Methods: Forty-two nondemented CAA patients, 50 HE subjects, and 43 AD/MCI patients had brain MRI and PiB PET. Multivariate linear regression was used to assess the independent association between PiB retention and white matter disease volume, controlling for age, gender, apolipoprotein E genotype, and vascular risk factors within each group.
Results: CAA patients were younger than HE and AD subjects (68 ± 10 vs 73.3 ± 7 and 74 ± 7.4, p < 0.01) but had higher amounts of WMH (median = 21 vs 3.2 and 10.8 ml, respectively, p < 0.05 for both comparisons). Global PiB retention and WMH showed strong correlation (rho = 0.52, p < 0.001) in the CAA group but not in HE or AD. These associations did not change in the multivariate models. Lobar microbleed count, another marker of CAA severity, also remained as an independent predictor of WMH volume.
Interpretation: Our results indicate that amyloid burden in CAA subjects (with primarily vascular amyloid) but not AD subjects (with primarily parenchymal amyloid) independently correlates with WMH volume. These findings support the idea that vascular amyloid burden directly contributes to chronic cerebral ischemia and highlights the possible utility of amyloid imaging as a marker of CAA severity.
Copyright © 2012 American Neurological Association.
Figures
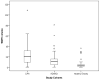
Figure 1. Comparison of WMH volume among the study cohorts
Comparison of WMH volume among the three diagnostic categories. Patients with CAA had higher WMH volume than HE (p<0.001) and AD/MCI (p=0.002) subjects. Patients with AD/MCI had higher WMH volume when compared to HE (p=0.01). These associations remained significant after adjustment for relevant covariates.
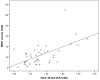
Figure 2. Scatterplot of PiB retention and WMH in cerebral amyloid angiopathy (CAA)
Scatterplot showing strong correlation between WMH and global DVR in cerebral amyloid angiopathy (CAA) (Spearman’s rho=0.52, p<0.001).
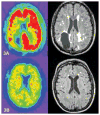
Figure 3. (A and B): Representative PiB PET and FLAIR MRIs showing correlation of amyloid deposition and WMH in 2 CAA patients
PiB PET (left) and FLAIR MRI (right) scans from 2 CAA patients with prior history of ICH, both had scans at 66 years of age. Both patients scored 30/30 on Mini-Mental Status Examination. Patient in panel 3A had a global DVR of 1.4 and WMH volume of 41ml. Patient in panel 3B had global DVR of 1.13 and WMH of 6.24ml.
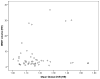
Figure 4. Scatterplot of PiB retention and WMH in patients with Alzheimer Disease (AD) and mild cognitive impairment (MCI)
Scatterplot showing no correlation between WMH and global DVR in patients with Alzheimer Disease (AD) and mild cognitive impairment (MCI) (Spearman’s rho=0.11, p=0.47).
Figure 5. Scatterplot of PiB retention and WMH in healthy elderly
Scatterplot showing no correlation between WMH and global DVR in healthy elderly (Spearman’s rho=0.01, p=0.95).
Similar articles
- Pathogenesis of cerebral microbleeds: In vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment.
Park JH, Seo SW, Kim C, Kim GH, Noh HJ, Kim ST, Kwak KC, Yoon U, Lee JM, Lee JW, Shin JS, Kim CH, Noh Y, Cho H, Kim HJ, Yoon CW, Oh SJ, Kim JS, Choe YS, Lee KH, Lee JH, Ewers M, Weiner MW, Werring DJ, Na DL. Park JH, et al. Ann Neurol. 2013 May;73(5):584-93. doi: 10.1002/ana.23845. Epub 2013 Mar 12. Ann Neurol. 2013. PMID: 23495089 - Imaging of amyloid burden and distribution in cerebral amyloid angiopathy.
Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE, Mathis CA, Price JC, Dekosky ST, Fischman AJ, Greenberg SM. Johnson KA, et al. Ann Neurol. 2007 Sep;62(3):229-34. doi: 10.1002/ana.21164. Ann Neurol. 2007. PMID: 17683091 - MRI-visible perivascular space location is associated with Alzheimer's disease independently of amyloid burden.
Banerjee G, Kim HJ, Fox Z, Jäger HR, Wilson D, Charidimou A, Na HK, Na DL, Seo SW, Werring DJ. Banerjee G, et al. Brain. 2017 Apr 1;140(4):1107-1116. doi: 10.1093/brain/awx003. Brain. 2017. PMID: 28335021 - Amyloid positron emission tomography in sporadic cerebral amyloid angiopathy: A systematic critical update.
Farid K, Charidimou A, Baron JC. Farid K, et al. Neuroimage Clin. 2017 May 5;15:247-263. doi: 10.1016/j.nicl.2017.05.002. eCollection 2017. Neuroimage Clin. 2017. PMID: 28560150 Free PMC article. Review. - Cerebral amyloid angiopathy in the elderly.
Viswanathan A, Greenberg SM. Viswanathan A, et al. Ann Neurol. 2011 Dec;70(6):871-80. doi: 10.1002/ana.22516. Ann Neurol. 2011. PMID: 22190361 Free PMC article. Review.
Cited by
- Brain White Matter: A Substrate for Resilience and a Substance for Subcortical Small Vessel Disease.
Sorond FA, Gorelick PB. Sorond FA, et al. Brain Sci. 2019 Aug 8;9(8):193. doi: 10.3390/brainsci9080193. Brain Sci. 2019. PMID: 31398858 Free PMC article. - Dissociating Statistically-Determined Alzheimer's Disease/Vascular Dementia Neuropsychological Syndromes Using White and Gray Neuroradiological Parameters.
Price CC, Tanner JJ, Schmalfuss IM, Brumback B, Heilman KM, Libon DJ. Price CC, et al. J Alzheimers Dis. 2015;48(3):833-47. doi: 10.3233/JAD-150407. J Alzheimers Dis. 2015. PMID: 26402109 Free PMC article. - White matter changes in dementia: role of impaired drainage of interstitial fluid.
Weller RO, Hawkes CA, Kalaria RN, Werring DJ, Carare RO. Weller RO, et al. Brain Pathol. 2015 Jan;25(1):63-78. doi: 10.1111/bpa.12218. Brain Pathol. 2015. PMID: 25521178 Free PMC article. Review. - Cerebral amyloid angiopathy: emerging concepts.
Yamada M. Yamada M. J Stroke. 2015 Jan;17(1):17-30. doi: 10.5853/jos.2015.17.1.17. Epub 2015 Jan 30. J Stroke. 2015. PMID: 25692104 Free PMC article. Review. - Regional white matter hyperintensities in posterior cortical atrophy and logopenic progressive aphasia.
Pham NTT, Graff-Radford J, Machulda MM, Spychalla AJ, Schwarz CG, Senjem ML, Lowe VJ, Vemuri P, Kantarci K, Knopman DS, Petersen RC, Jack CR, Josephs KA, Whitwell JL. Pham NTT, et al. Neurobiol Aging. 2022 Nov;119:46-55. doi: 10.1016/j.neurobiolaging.2022.07.008. Epub 2022 Jul 28. Neurobiol Aging. 2022. PMID: 35970009 Free PMC article.
References
- Oksala NK, Oksala A, Pohjasvaara T, et al. Age related white matter changes predict stroke death in long term follow-up. Journal of neurology, neurosurgery, and psychiatry. 2009;80:762–766. - PubMed
- Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. - PubMed
- Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–942. - PubMed
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG026484/AG/NIA NIH HHS/United States
- T32 NS048005/NS/NINDS NIH HHS/United States
- R01 NS070834/NS/NINDS NIH HHS/United States
- K01 AG040197/AG/NIA NIH HHS/United States
- T32NS048005/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical