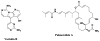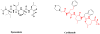Natural products: a continuing source of novel drug leads - PubMed (original) (raw)
Review
Natural products: a continuing source of novel drug leads
Gordon M Cragg et al. Biochim Biophys Acta. 2013 Jun.
Abstract
Background: Nature has been a source of medicinal products for millennia, with many useful drugs developed from plant sources. Following discovery of the penicillins, drug discovery from microbial sources occurred and diving techniques in the 1970s opened the seas. Combinatorial chemistry (late 1980s), shifted the focus of drug discovery efforts from Nature to the laboratory bench.
Scope of review: This review traces natural products drug discovery, outlining important drugs from natural sources that revolutionized treatment of serious diseases. It is clear Nature will continue to be a major source of new structural leads, and effective drug development depends on multidisciplinary collaborations.
Major conclusions: The explosion of genetic information led not only to novel screens, but the genetic techniques permitted the implementation of combinatorial biosynthetic technology and genome mining. The knowledge gained has allowed unknown molecules to be identified. These novel bioactive structures can be optimized by using combinatorial chemistry generating new drug candidates for many diseases.
General significance: The advent of genetic techniques that permitted the isolation / expression of biosynthetic cassettes from microbes may well be the new frontier for natural products lead discovery. It is now apparent that biodiversity may be much greater in those organisms. The numbers of potential species involved in the microbial world are many orders of magnitude greater than those of plants and multi-celled animals. Coupling these numbers to the number of currently unexpressed biosynthetic clusters now identified (>10 per species) the potential of microbial diversity remains essentially untapped.
Published by Elsevier B.V.
Figures
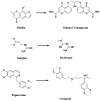
Fig. 1
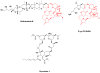
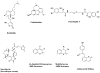
Drugs based on traditional medicine leads (khellin, sodium chromoglycate, galegine, metformin, papaverine, verapamil)
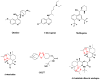
Fig. 2
Natural antimalarial agents and analogues Quinine, chloroquine, mefloquine, artemisinin, OZ277, Dimeric analogue
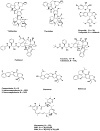
Fig. 3
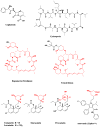
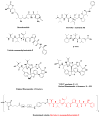
Plant-derived anticancer agents Vinblastine / vincristine, Etoposide, Paclitaxel, Taxotere, Cabazitaxel, Camptothecin / 9-NH2, 9-NO2 / Topotecan / Irinotecan / Belotecan, Maytansine
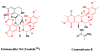
Fig. 4
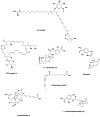
Ecteinascidin 743 (Yondelis®) and its semisynthetic precursor
Fig. 5
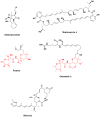
Halichondrin B, Eribulin, Bryostatin 1
Fig. 6
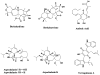
Drugs from Microbes Cephalosporins, cyclosporins, rapamycin, statins
Fig. 7
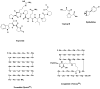
Microbial-Derived Anticancer Agents Daunomycin, bleomycin A2, mitomycin C, calicheamicin
Fig. 8
Epothilone anticancer agents Epothilones A–D, ixabepilone, sagopilone didehydroepothilone D, isoxazolefludelone
Fig. 9
Chitin and Glucan Inhibitors Nikkomycin Z, Caspofungin, Anidulafungin, Micafungin
Fig. 10
Drugs from amphibian, reptilian and human sources Teprotide, captopril, epibatidine, Byetta®, liraglutide
Fig. 11
Sources of Drugs
Fig 12
Natural products and the cell cycle
Fig. 13
Natural Products from Antarctic sources Variolins, Palmerolide
Fig 14
Natural products from heterologous gene expression, extremophiles and endophytes Pantocin, berkeleydione, berkeleytrione, ambuic acid, aspochalasins, terrequinone
Fig. 15
Examples of novel microbial natural products Salinosporamide, marinomycins A-D, maytansine, pederin, onnamide, rhizoxin
Fig. 16
Products of Total Synthesis Diazonamide, discodermolide, TZT-1027/auristatin PE, E-7974
Fig. 17
Products of Diversity-Oriented and Parallel Synthesis and Privileged Structures Dysidiolide, galanthamine, psammaplin, sarcodictyin, 2,2-dimethyl-2H-benzopyran, benzopyrans plus cyanostilbene substitution
Fig. 18
New compounds from a variety of approaches ECO 0501, chivosazol, platensimycin, platencin, phomallenic acid C, lucensimycin A, (−)-adamantaplatensimycin
Fig. 19
Carfilzomib, Epoxomicin
Similar articles
- Natural product discovery: past, present, and future.
Katz L, Baltz RH. Katz L, et al. J Ind Microbiol Biotechnol. 2016 Mar;43(2-3):155-76. doi: 10.1007/s10295-015-1723-5. Epub 2016 Jan 6. J Ind Microbiol Biotechnol. 2016. PMID: 26739136 Review. - Discovery of novel bioactive natural products driven by genome mining.
Li Z, Zhu D, Shen Y. Li Z, et al. Drug Discov Ther. 2018;12(6):318-328. doi: 10.5582/ddt.2018.01066. Drug Discov Ther. 2018. PMID: 30674766 Review. - Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019.
Newman DJ, Cragg GM. Newman DJ, et al. J Nat Prod. 2020 Mar 27;83(3):770-803. doi: 10.1021/acs.jnatprod.9b01285. Epub 2020 Mar 12. J Nat Prod. 2020. PMID: 32162523 Review. - Medicinals for the millennia: the historical record.
Cragg GM, Newman DJ. Cragg GM, et al. Ann N Y Acad Sci. 2001 Dec;953:3-25. doi: 10.1111/j.1749-6632.2001.tb11356.x. Ann N Y Acad Sci. 2001. PMID: 11795420 - Natural product drug discovery in the next millennium.
Cragg GM, Newman DJ. Cragg GM, et al. Pharm Biol. 2001;39 Suppl 1:8-17. doi: 10.1076/phbi.39.s1.8.0009. Pharm Biol. 2001. PMID: 21554167
Cited by
- Progress in the Stereoselective Synthesis Methods of Pyrrolidine-Containing Drugs and Their Precursors.
Smolobochkin A, Gazizov A, Appazov N, Sinyashin O, Burilov A. Smolobochkin A, et al. Int J Mol Sci. 2024 Oct 17;25(20):11158. doi: 10.3390/ijms252011158. Int J Mol Sci. 2024. PMID: 39456938 Free PMC article. Review. - Fragment-Based Drug Discovery against Mycobacteria: The Success and Challenges.
Togre NS, Vargas AM, Bhargavi G, Mallakuntla MK, Tiwari S. Togre NS, et al. Int J Mol Sci. 2022 Sep 14;23(18):10669. doi: 10.3390/ijms231810669. Int J Mol Sci. 2022. PMID: 36142582 Free PMC article. Review. - Resveratrol-loaded nanomedicines for cancer applications.
Annaji M, Poudel I, Boddu SHS, Arnold RD, Tiwari AK, Babu RJ. Annaji M, et al. Cancer Rep (Hoboken). 2021 Jun;4(3):e1353. doi: 10.1002/cnr2.1353. Epub 2021 Mar 2. Cancer Rep (Hoboken). 2021. PMID: 33655717 Free PMC article. Review. - Cornulacin: a new isoflavone from Cornulaca monacantha and its isolation, structure elucidation and cytotoxicity through EGFR-mediated apoptosis.
Badawy AM, Eltamany EE, Hussien RM, Mohamed OG, El-Ayouty MM, Nafie MS, Tripathi A, Ahmed SA. Badawy AM, et al. RSC Med Chem. 2024 Aug 22;15(9):3228-38. doi: 10.1039/d4md00524d. Online ahead of print. RSC Med Chem. 2024. PMID: 39185453 Free PMC article. - Saraca indica bark extract shows in vitro antioxidant, antibreast cancer activity and does not exhibit toxicological effects.
Yadav NK, Saini KS, Hossain Z, Omer A, Sharma C, Gayen JR, Singh P, Arya KR, Singh RK. Yadav NK, et al. Oxid Med Cell Longev. 2015;2015:205360. doi: 10.1155/2015/205360. Epub 2015 Mar 16. Oxid Med Cell Longev. 2015. PMID: 25861411 Free PMC article.
References
- Borchardt JK. The beginnings of drug therapy: Ancient mesopotamian medicine. Drug News Perspect. 2002;15:187–192. - PubMed
- Huang KC. The pharmacology of chinese herbs. 2. CRC Press; Boca Raton, FL: 1999.
- Kapoor LD. Crc handbook of ayurvedic medicinal plants. CRC Press; Boca Raton, FL: 1990.
- Moerman DE. Medicinal plants of native america. University of Michigan Museum of Anthropology; Ann Arbor, MI: 1986.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials