A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease - PubMed (original) (raw)
doi: 10.1371/journal.pone.0056695. Epub 2013 Feb 19.
Hitoshi Sugiyama, Hiroshi Morinaga, Tatsuyuki Inoue, Keiichi Takiue, Ayu Ogawa, Toshio Yamanari, Yoko Kikumoto, Haruhito Adam Uchida, Shinji Kitamura, Yohei Maeshima, Kazufumi Nakamura, Hiroshi Ito, Hirofumi Makino
Affiliations
- PMID: 23431388
- PMCID: PMC3576368
- DOI: 10.1371/journal.pone.0056695
A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease
Masashi Kitagawa et al. PLoS One. 2013.
Abstract
Background: Klotho was originally identified in a mutant mouse strain unable to express the gene that consequently showed shortened life spans. In humans, low serum Klotho levels are related to the prevalence of cardiovascular diseases in community-dwelling adults. However, it is unclear whether the serum Klotho levels are associated with signs of vascular dysfunction such as arterial stiffness, a major determinant of prognosis, in human subjects with chronic kidney disease (CKD).
Methods: We determined the levels of serum soluble Klotho in 114 patients with CKD using ELISA and investigated the relationship between the level of Klotho and markers of CKD-mineral and bone disorder (CKD-MBD) and various types of vascular dysfunction, including flow-mediated dilatation, a marker of endothelial dysfunction, ankle-brachial pulse wave velocity (baPWV), a marker of arterial stiffness, intima-media thickness (IMT), a marker of atherosclerosis, and the aortic calcification index (ACI), a marker of vascular calcification.
Results: The serum Klotho level significantly correlated with the 1,25-dihydroxyvitamin D level and inversely correlated with the parathyroid hormone level and the fractional excretion of phosphate. There were significant decreases in serum Klotho in patients with arterial stiffness defined as baPWV≥1400 cm/sec, atherosclerosis defined as maximum IMT≥1.1 mm and vascular calcification scores of ACI>0%. The serum Klotho level was a significant determinant of arterial stiffness, but not endothelial dysfunction, atherosclerosis or vascular calcification, in the multivariate analysis in either metabolic model, the CKD model or the CKD-MBD model. The adjusted odds ratio of serum Klotho for the baPWV was 0.60 (p = 0.0075).
Conclusions: Decreases in the serum soluble Klotho levels are independently associated with signs of vascular dysfunction such as arterial stiffness in patients with CKD. Further research exploring whether therapeutic approaches to maintain or elevate the Klotho level could improve arterial stiffness in CKD patients is warranted.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
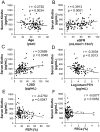
Figure 1. Correlation between the serum Klotho levels (pg/mL) and various parameters.
The relationships between the serum Klotho levels and patient age (years) (A), estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) (B) and markers of chronic kidney disease-mineral and bone disorder (CKD-MBD), including 1,25-dihydroxyvitamin D (1,25D) (pg/mL) (C), log intact parathyroid hormone (PTH) (pg/mL) (D), fractional excretion of phosphate (FEPi) (%) (E) and fractional excretion of calcium (FECa) (%) (F) are shown. The serum Klotho levels were inversely correlated with age and positively correlated with eGFR (A, B). Regarding CKD-MBD markers, the serum Klotho levels were significantly correlated with 1,25D and negatively correlated with log intact PTH and FEPi; however, no significant correlation was observed with FECa (C–F). (A–F) N = 114.
Figure 2. Box and line plots showing the levels of serum Klotho (pg/mL) according to the stratified levels of vascular dysfunction.
They include flow-mediated dilatation (FMD) (%), a marker of endothelial dysfunction (A), ankle-brachial pulse wave velocity (baPWV) (cm/sec), a marker of arterial stiffness (B), maximum intima-media thickness (max IMT) (mm), a marker of atherosclerosis (C), and the aortic calcification index (ACI) (%), a marker of vascular calcification (D). The serum Klotho levels were significantly lower in patients with FMD<6.0%, PWV≥1400 cm/s, max IMT≥1.1 mm and ACI>0% compared to patients with FMD≥6.0%, PWV<1400 cm/s, max IMT<1.1 mm and ACI = 0%, respectively (**A–D**). (**A**) N = 70 and n = 40 in FMD<6.0% and FMD≥6.0%, respectively. (**B**) N = 60 and n = 45 in PWV<1400 cm/s and PWV≥1400 cm/s, respectively. (**C**) N = 82 and n = 29 in max IMT<1.1 mm and max IMT≥1.1 mm, respectively. (**D**) N = 28 and n = 75 in ACI = 0% and ACI>0%, respectively. The boxes denote the medians and 25th and 75th percentiles. The lines mark the 5th and 95th percentiles.
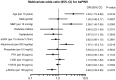
Figure 3. Multivariate odds ratio for ankle-brachial pulse wave velocity (baPWV) among patients with CKD displayed as the odds ratio (OR) (solid boxes) with 95% confidence intervals (CIs) (horizontal limit lines).
For continuous variables, the unit of change is given in parenthesis based on the multivariate model described in Table 2. MBP, mean blood pressure; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone; 1,25D, 1,25-dihydroxyvitamin D; FGF23, fibroblast growth factor 23.
Similar articles
- The association between soluble klotho and cardiovascular parameters in chronic kidney disease: results from the KNOW-CKD study.
Kim HJ, Kang E, Oh YK, Kim YH, Han SH, Yoo TH, Chae DW, Lee J, Ahn C, Oh KH. Kim HJ, et al. BMC Nephrol. 2018 Mar 5;19(1):51. doi: 10.1186/s12882-018-0851-3. BMC Nephrol. 2018. PMID: 29506503 Free PMC article. Clinical Trial. - [Role of the morphogenetic proteins FGF-23 and Klotho and the glycoprotein sclerostin in the assessment of the risk of cardiovascular diseases and the prognosis of chronic kidney disease].
Milovanova LY, Milovanov YS, Kudryavtseva DV, Markina MM, Milovanova SY, Kozlovskaya LV, Lebedeva MV, Beketov VD, Moiseev SV, Mukhin NA, Fomin VV, Svistunov AA. Milovanova LY, et al. Ter Arkh. 2015;87(6):10-16. doi: 10.17116/terarkh201587610-16. Ter Arkh. 2015. PMID: 26281189 Russian. - Progression of arterial stiffness is associated with changes in bone mineral markers in advanced CKD.
Krishnasamy R, Tan SJ, Hawley CM, Johnson DW, Stanton T, Lee K, Mudge DW, Campbell S, Elder GJ, Toussaint ND, Isbel NM. Krishnasamy R, et al. BMC Nephrol. 2017 Sep 4;18(1):281. doi: 10.1186/s12882-017-0705-4. BMC Nephrol. 2017. PMID: 28870151 Free PMC article. - FGF23 and Klotho in chronic kidney disease.
Olauson H, Larsson TE. Olauson H, et al. Curr Opin Nephrol Hypertens. 2013 Jul;22(4):397-404. doi: 10.1097/MNH.0b013e32836213ee. Curr Opin Nephrol Hypertens. 2013. PMID: 23666415 Review.
Cited by
- Implications of Klotho in vascular health and disease.
Martín-Núñez E, Donate-Correa J, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. Martín-Núñez E, et al. World J Cardiol. 2014 Dec 26;6(12):1262-9. doi: 10.4330/wjc.v6.i12.1262. World J Cardiol. 2014. PMID: 25548616 Free PMC article. Review. - Uremic Toxins and Vascular Dysfunction.
Six I, Flissi N, Lenglet G, Louvet L, Kamel S, Gallet M, Massy ZA, Liabeuf S. Six I, et al. Toxins (Basel). 2020 Jun 18;12(6):404. doi: 10.3390/toxins12060404. Toxins (Basel). 2020. PMID: 32570781 Free PMC article. Review. - Effects of Eicosapentaenoic Acid on Arterial Calcification.
Saito Y, Nakamura K, Ito H. Saito Y, et al. Int J Mol Sci. 2020 Jul 30;21(15):5455. doi: 10.3390/ijms21155455. Int J Mol Sci. 2020. PMID: 32751754 Free PMC article. Review. - Klotho suppresses cardiomyocyte apoptosis in mice with stress-induced cardiac injury via downregulation of endoplasmic reticulum stress.
Song S, Gao P, Xiao H, Xu Y, Si LY. Song S, et al. PLoS One. 2013 Dec 10;8(12):e82968. doi: 10.1371/journal.pone.0082968. eCollection 2013. PLoS One. 2013. PMID: 24340070 Free PMC article. - Klotho recovery by genistein via promoter histone acetylation and DNA demethylation mitigates renal fibrosis in mice.
Li Y, Chen F, Wei A, Bi F, Zhu X, Yin S, Lin W, Cao W. Li Y, et al. J Mol Med (Berl). 2019 Apr;97(4):541-552. doi: 10.1007/s00109-019-01759-z. Epub 2019 Feb 26. J Mol Med (Berl). 2019. PMID: 30806715
References
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, et al. (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169. - PubMed
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305. - PubMed
- Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, et al. (2006) Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953. - PubMed
- KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl: S1–130. - PubMed
MeSH terms
Substances
Grants and funding
A portion of this study was supported by a Research Grant from the Kidney Foundation Japan (JKFB10-20 and JKFB12-44) and a 2011 Chronic Kidney Disease Award to M.K., a Research Grant from the Japan Vascular Disease Research Foundation to H.S. and a Grant-in-Aid for Progressive Renal Diseases Research, Research on Intractable Disease, from the Ministry of Health, Labor and Welfare of Japan. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical