Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs - PubMed (original) (raw)
Review
Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs
Samy Lamouille et al. Curr Opin Cell Biol. 2013 Apr.
Abstract
Epithelial-mesenchymal transition (EMT) and the reverse process, mesenchymal-epithelial transition (MET), are essential during development and in the regulation of stem cell pluripotency, yet these processes are also activated in pathological contexts, such as in fibrosis and cancer progression. In EMT and MET, diverse signaling pathways cooperate in the initiation and progression of the EMT and MET programs, through regulation at transcriptional, post-transcriptional, translational, and post-translational levels. MicroRNAs recently emerged as potent regulators of EMT and MET, with their abilities to target multiple components involved in epithelial integrity or mesenchymal traits. By affecting EMT and MET processes, microRNAs are involved in the regulation of stem cell pluripotency and the control of tumor progression.
Copyright © 2013. Published by Elsevier Ltd.
Figures
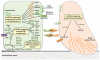
Figure 1
MicroRNAs in EMT and MET. EMT is characterized by a disassembly of cell–cell junctions, loss of epithelial polarity, and reorganization of actin cytoskeleton. In addition to a decrease in epithelial marker expression, increases in expression of mesenchymal markers and invasive behavior are observed in cells undergoing EMT. The changes in gene expression programs are activated by families of transcription factors. MicroRNAs suppress production of these transcription factors as well as multiple markers and components that define the epithelial or mesenchymal characteristics. These microRNAs can therefore promote EMT (orange) or repress EMT and enhance MET programs (green).
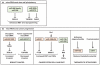
Figure 2
Different mechanisms of action of microRNAs in regulating EMT. Three distinct mechanisms of regulation of EMT components by microRNAs can be discerned. (a) Two distinct microRNAs can cooperate to regulate the expression of a single transcript by targeting different sites in the transcript’s 3′UTR. (b) One single microRNA can regulate multiple EMT components. (c) Double feedback loops are observed when the expression of microRNA is targeted and downregulated by its own EMT transcription factor target.
Figure 3
MicroRNAs regulate EMT-associated and MET-associated stem cell pluripotency and cancer progression. (a) The induction of pluripotent stem cells requires the acquisition of epithelial characteristics and is initiated by an MET event. By inhibiting EMT and enhancing MET programs (green), microRNAs can enhance reprogramming and stem cell pluripotency. (b) EMT and MET programs have been associated with cancer progression. Therefore, microRNAs that regulate EMT or MET programs can present oncogenic (orange) or tumor-suppressive roles (green). The presence of cancer stem cells that arise from EMT events within a population of tumor cells can also be regulated by the action of microRNAs. Therefore, blocking oncogenic microRNAs or expressing tumor-suppressive microRNAs represent potent strategies against tumor progression.
Similar articles
- Incomplete cellular reprogramming of colorectal cancer cells elicits an epithelial/mesenchymal hybrid phenotype.
Hiew MSY, Cheng HP, Huang CJ, Chong KY, Cheong SK, Choo KB, Kamarul T. Hiew MSY, et al. J Biomed Sci. 2018 Jul 19;25(1):57. doi: 10.1186/s12929-018-0461-1. J Biomed Sci. 2018. PMID: 30025541 Free PMC article. - Regulation of mesenchymal phenotype by MicroRNAs in cancer.
Yan J, Gumireddy K, Li A, Huang Q. Yan J, et al. Curr Cancer Drug Targets. 2013 Nov;13(9):930-4. doi: 10.2174/15680096113136660098. Curr Cancer Drug Targets. 2013. PMID: 24168190 Free PMC article. Review. - Transcriptional and post-transcriptional control of epithelial-mesenchymal plasticity: why so many regulators?
Migault M, Sapkota S, Bracken CP. Migault M, et al. Cell Mol Life Sci. 2022 Mar 12;79(3):182. doi: 10.1007/s00018-022-04199-0. Cell Mol Life Sci. 2022. PMID: 35278142 Free PMC article. Review. - MicroRNA-Mediated Post-Transcriptional Regulation of Epithelial to Mesenchymal Transition in Cancer.
Behbahani GD, Ghahhari NM, Javidi MA, Molan AF, Feizi N, Babashah S. Behbahani GD, et al. Pathol Oncol Res. 2017 Jan;23(1):1-12. doi: 10.1007/s12253-016-0101-6. Epub 2016 Sep 2. Pathol Oncol Res. 2017. PMID: 27590333 Review. - MicroRNAs as critical regulators involved in regulating epithelial- mesenchymal transition.
Zhao X, Lu Y, Nie Y, Fan D. Zhao X, et al. Curr Cancer Drug Targets. 2013 Nov;13(9):935-44. doi: 10.2174/15680096113136660099. Curr Cancer Drug Targets. 2013. PMID: 24168189 Review.
Cited by
- Relationship of Signaling Pathways between RKIP Expression and the Inhibition of EMT-Inducing Transcription Factors SNAIL1/2, TWIST1/2 and ZEB1/2.
Bustamante A, Baritaki S, Zaravinos A, Bonavida B. Bustamante A, et al. Cancers (Basel). 2024 Sep 17;16(18):3180. doi: 10.3390/cancers16183180. Cancers (Basel). 2024. PMID: 39335152 Free PMC article. Review. - How early life respiratory viral infections impact airway epithelial development and may lead to asthma.
Berdnikovs S, Newcomb DC, Hartert TV. Berdnikovs S, et al. Front Pediatr. 2024 Aug 2;12:1441293. doi: 10.3389/fped.2024.1441293. eCollection 2024. Front Pediatr. 2024. PMID: 39156016 Free PMC article. Review. - Mitochondrial Ca2+ controls pancreatic cancer growth and metastasis by regulating epithelial cell plasticity.
Weissenrieder JS, Peura J, Paudel U, Bhalerao N, Weinmann N, Johnson C, Wengyn M, Drager R, Furth EE, Simin K, Ruscetti M, Stanger BZ, Rustgi AK, Pitarresi JR, Foskett JK. Weissenrieder JS, et al. bioRxiv [Preprint]. 2024 Aug 9:2024.08.08.607195. doi: 10.1101/2024.08.08.607195. bioRxiv. 2024. PMID: 39149344 Free PMC article. Preprint. - Expression Profiles of Dopamine-Related Genes and miRNAs Regulating Their Expression in Breast Cancer.
Sirek T, Sirek A, Borawski P, Ryguła I, Król-Jatręga K, Opławski M, Boroń D, Chalcarz M, Ossowski P, Dziobek K, Zmarzły N, Boroń K, Mickiewicz P, Grabarek BO. Sirek T, et al. Int J Mol Sci. 2024 Jun 14;25(12):6546. doi: 10.3390/ijms25126546. Int J Mol Sci. 2024. PMID: 38928253 Free PMC article. - Dissecting the multifaceted roles of autophagy in cancer initiation, growth, and metastasis: from molecular mechanisms to therapeutic applications.
Ayub A, Hasan MK, Mahmud Z, Hossain MS, Kabir Y. Ayub A, et al. Med Oncol. 2024 Jun 20;41(7):183. doi: 10.1007/s12032-024-02417-2. Med Oncol. 2024. PMID: 38902544 Review.
References
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. - PubMed
- Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res. 2010;8:629–642. - PubMed
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous