Quantitative description of glycan-receptor binding of influenza A virus H7 hemagglutinin - PubMed (original) (raw)
Quantitative description of glycan-receptor binding of influenza A virus H7 hemagglutinin
Karunya Srinivasan et al. PLoS One. 2013.
Erratum in
- PLoS One. 2013;8(12). doi:10.1371/annotation/c3b40a6f-a4be-4117-8ce2-a1b9b873e87c
Abstract
In the context of recently emerged novel influenza strains through reassortment, avian influenza subtypes such as H5N1, H7N7, H7N2, H7N3 and H9N2 pose a constant threat in terms of their adaptation to the human host. Among these subtypes, it was recently demonstrated that mutations in H5 and H9 hemagglutinin (HA) in the context of lab-generated reassorted viruses conferred aerosol transmissibility in ferrets (a property shared by human adapted viruses). We previously demonstrated that the quantitative binding affinity of HA to α2→6 sialylated glycans (human receptors) is one of the important factors governing human adaptation of HA. Although the H7 subtype has infected humans causing varied clinical outcomes from mild conjunctivitis to severe respiratory illnesses, it is not clear where the HA of these subtypes stand in regard to human adaptation since its binding affinity to glycan receptors has not yet been quantified. In this study, we have quantitatively characterized the glycan receptor-binding specificity of HAs from representative strains of Eurasian (H7N7) and North American (H7N2) lineages that have caused human infection. Furthermore, we have demonstrated for the first time that two specific mutations; Gln226→Leu and Gly228→Ser in glycan receptor-binding site of H7 HA substantially increase its binding affinity to human receptor. Our findings contribute to a framework for monitoring the evolution of H7 HA to be able to adapt to human host.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
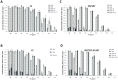
Figure 1. Glycan receptor-binding specificity of FC, CC, NY/107 and mNY/107:A135T HA.
A, shows dose-dependent direct glycan array binding of FC HA. Specific high affinity binding to avian receptors (3′ SLN, 3′ SLNLN and 3′ SLNLNLN) and no binding to human receptors is observed. B, shows dose-dependent direct glycan array binding of CC HA. High affinity binding to avian receptors is observed. In comparison with FC, there is observable binding to human receptors (6′SLN-LN and 6′SLN) albeit at orders of magnitude lower affinity than binding to avian receptors. C, shows dose-dependent direct glycan array binding of NY/107 HA. High affinity binding to avian receptors (3′SLN-LN and 3′SLN-LN-LN) is observed with binding affinity for 3′SLN lower than that of FC and CC HAs. Binding to human receptor is observed but at much lower affinity (by orders of magnitude) than binding to avian receptors. D, shows dose-dependent direct glycan array binding of mutant mNY/107:A135T HA. Introduction of glycosylation sequon at Asn-133 does not seem to alter binding of this mutant HA in relation to the wild type.
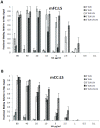
Figure 2. Glycan receptor-binding specificity of Gln226→Leu/Gly228→Ser mutant of FC and CC.
A and B, respectively show dose-dependent direct glycan array binding of mFC: LS and mCC: LS mutant HAs. The double mutation leads to a substantial increase in human-receptor binding signals to a level that allowed calculation of apparent binding affinity parameter Kd'. The double mutation also lowers the avian-receptor binding affinity of mutant HAs relative to the corresponding wild-type HAs.
Figure 3. Structural complexes of FC – avian receptor and mFC:LS – human receptor.
A, shows the structural complex of RBS of FC HA with avian receptor derived from the X-ray co-crystal structure of this complex (PDB ID: 4DJ7). B, shows structural complex of RBS of mFC:LS HA with human receptor derived based on homology-model of mFC:LS (generated using automated mode in Swiss Model:
). The coordinates of the human receptor were obtained from PDB ID: 2WR7. The mFC:LS human receptor structural complex was obtained by superimposing the HA1 domain (bound to this receptor) of 2WR7 with that of mFC:LS HA1 homology model followed by subsequent energy minimization. The critical contacts made by Gln-226 (in FC HA) and Leu-226 (in mFC:LS HA) with glycan receptor is shown using a dotted line.
Figure 4. Tissue binding specificity of FC and mFC:LS for human tracheal and alveolar sections.
A, Extensive staining of apical surface of human tracheal epithelia for the mFC: LS (green) against propidium iodide staining (in red) is observed. Bright staining of what appears to be goblet cells (inset at 20x magnification; indicated by white arrow) by this mutant HA resembles a similar pattern that was previously observed with 1918 H1N1 and 1958 H2N2 HAs. B, Shows minimal to no staining of apical surface of tracheal section by FC consistent with its low binding to human receptors on glycan array. C and D respectively show intense staining of alveolar section by mFC:LS and FC consistent with their high affinity binding to avian receptors.
Similar articles
- Quantitative characterization of glycan-receptor binding of H9N2 influenza A virus hemagglutinin.
Srinivasan K, Raman R, Jayaraman A, Viswanathan K, Sasisekharan R. Srinivasan K, et al. PLoS One. 2013 Apr 23;8(4):e59550. doi: 10.1371/journal.pone.0059550. Print 2013. PLoS One. 2013. PMID: 23626667 Free PMC article. - Enhanced Human-Type Receptor Binding by Ferret-Transmissible H5N1 with a K193T Mutation.
Peng W, Bouwman KM, McBride R, Grant OC, Woods RJ, Verheije MH, Paulson JC, de Vries RP. Peng W, et al. J Virol. 2018 Apr 27;92(10):e02016-17. doi: 10.1128/JVI.02016-17. Print 2018 May 15. J Virol. 2018. PMID: 29491160 Free PMC article. - Receptor binding by an H7N9 influenza virus from humans.
Xiong X, Martin SR, Haire LF, Wharton SA, Daniels RS, Bennett MS, McCauley JW, Collins PJ, Walker PA, Skehel JJ, Gamblin SJ. Xiong X, et al. Nature. 2013 Jul 25;499(7459):496-9. doi: 10.1038/nature12372. Nature. 2013. PMID: 23787694 - Adaptation of influenza viruses to human airway receptors.
Thompson AJ, Paulson JC. Thompson AJ, et al. J Biol Chem. 2021 Jan-Jun;296:100017. doi: 10.1074/jbc.REV120.013309. Epub 2020 Nov 22. J Biol Chem. 2021. PMID: 33144323 Free PMC article. Review. - Receptor binding properties of the influenza virus hemagglutinin as a determinant of host range.
Xiong X, McCauley JW, Steinhauer DA. Xiong X, et al. Curr Top Microbiol Immunol. 2014;385:63-91. doi: 10.1007/82_2014_423. Curr Top Microbiol Immunol. 2014. PMID: 25078920 Review.
Cited by
- Avian influenza.
EFSA Panel on Animal Health and Welfare (AHAW); More S, Bicout D, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin-Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Thulke HH, Velarde A, Willeberg P, Winckler C, Breed A, Brouwer A, Guillemain M, Harder T, Monne I, Roberts H, Baldinelli F, Barrucci F, Fabris C, Martino L, Mosbach-Schulz O, Verdonck F, Morgado J, Stegeman JA. EFSA Panel on Animal Health and Welfare (AHAW), et al. EFSA J. 2017 Oct 16;15(10):e04991. doi: 10.2903/j.efsa.2017.4991. eCollection 2017 Oct. EFSA J. 2017. PMID: 32625288 Free PMC article. - The Epidemiology, Virology, and Pathogenicity of Human Infections with Avian Influenza Viruses.
Wang D, Zhu W, Yang L, Shu Y. Wang D, et al. Cold Spring Harb Perspect Med. 2021 Apr 1;11(4):a038620. doi: 10.1101/cshperspect.a038620. Cold Spring Harb Perspect Med. 2021. PMID: 31964651 Free PMC article. Review. - A study of the relationship between human infection with avian influenza a (H5N6) and environmental avian influenza viruses in Fujian, China.
Chen P, Xie JF, Lin Q, Zhao L, Zhang YH, Chen HB, Weng YW, Huang Z, Zheng KC. Chen P, et al. BMC Infect Dis. 2019 Sep 2;19(1):762. doi: 10.1186/s12879-019-4145-6. BMC Infect Dis. 2019. PMID: 31477028 Free PMC article. - Inventory of molecular markers affecting biological characteristics of avian influenza A viruses.
Suttie A, Deng YM, Greenhill AR, Dussart P, Horwood PF, Karlsson EA. Suttie A, et al. Virus Genes. 2019 Dec;55(6):739-768. doi: 10.1007/s11262-019-01700-z. Epub 2019 Aug 19. Virus Genes. 2019. PMID: 31428925 Free PMC article. Review. - The re-emergence of highly pathogenic avian influenza H7N9 viruses in humans in mainland China, 2019.
Yu D, Xiang G, Zhu W, Lei X, Li B, Meng Y, Yang L, Jiao H, Li X, Huang W, Wei H, Zhang Y, Hai Y, Zhang H, Yue H, Zou S, Zhao X, Li C, Ao D, Zhang Y, Tan M, Liu J, Zhang X, Gao GF, Meng L, Wang D. Yu D, et al. Euro Surveill. 2019 May;24(21):1900273. doi: 10.2807/1560-7917.ES.2019.24.21.1900273. Euro Surveill. 2019. PMID: 31138362 Free PMC article.
References
- Malik Peiris JS (2009) Avian influenza viruses in humans. Revue scientifique et technique 28: 161–173. - PubMed
- Ge S, Wang Z (2011) An overview of influenza A virus receptors. Critical reviews in microbiology 37: 157–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous