Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults - PubMed (original) (raw)
Review
Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults
Karen R Steingart et al. Cochrane Database Syst Rev. 2013.
Update in
- Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults.
Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Steingart KR, et al. Cochrane Database Syst Rev. 2014 Jan 21;2014(1):CD009593. doi: 10.1002/14651858.CD009593.pub3. Cochrane Database Syst Rev. 2014. PMID: 24448973 Free PMC article. Updated. Review.
Abstract
Background: Accurate and rapid detection of tuberculosis (TB) and drug resistance are critical for improving patient care and decreasing the spread of TB. Xpert® MTB/RIF assay (Xpert) is a rapid, automated test that can detect both TB and rifampicin resistance, within two hours after starting the test, with minimal hands-on technical time, but is more expensive than conventional sputum microscopy.
Objectives: To assess the diagnostic accuracy of Xpert for pulmonary TB (TB detection), both where Xpert was used as an initial test replacing microscopy, and where Xpert was used as an add-on test following a negative smear microscopy result.To assess the diagnostic accuracy of Xpert for rifampicin resistance detection where Xpert was used as the initial test, replacing conventional culture-based drug susceptibility testing.The population of interest was adults suspected of having pulmonary TB or multidrug-resistant TB (MDR-TB), with or without HIV infection.
Search methods: We performed a comprehensive search of the following databases: Cochrane Infectious Diseases Group Specialized Register; MEDLINE; EMBASE; ISI Web of Knowledge; MEDION; LILACS; BIOSIS; and SCOPUS. We also searched the metaRegister of Controlled Trials (mRCT) and the search portal of the WHO International Clinical Trials Registry Platform to identify ongoing trials. We performed searches on 25 September 2011 and we repeated them on 15 December 2011, without language restriction.
Selection criteria: We included randomized controlled trials, cross-sectional, and cohort studies that used respiratory specimens to compare Xpert with culture for detecting TB and Xpert with conventional phenotypic drug susceptibility testing for detecting rifampicin resistance.
Data collection and analysis: For each study, two review authors independently extracted a set of data using a standardized data extraction form. When possible, we extracted data for subgroups by smear and HIV status. We assessed the quality of studies using the QUADAS-2 tool. We carried out meta-analyses to estimate the pooled sensitivity and specificity of Xpert separately for TB detection and rifampicin resistance detection using a bivariate random-effects model. We estimated the median pooled sensitivity and specificity and their 95% credible intervals (CrI).
Main results: We identified 18 unique studies as eligible for this review, including two multicentre international studies, one with five and the other with six distinct study centres. The majority of studies (55.6%) were performed in low-income and middle-income countries. In 17 of the 18 studies, Xpert was performed by trained technicians in reference laboratories.When used as an initial test replacing smear microscopy (15 studies, 7517 participants), Xpert achieved a pooled sensitivity of 88% (95% CrI 83% to 92%) and pooled specificity of 98% (95% CrI 97% to 99%). As an add-on test following a negative smear microscopy result (14 studies, 5719 participants), Xpert yielded a pooled sensitivity of 67% (95% CrI 58% to 74%) and pooled specificity of 98% (95% CrI 97% to 99%). In clinical subgroups, we found the following accuracy estimates: the pooled sensitivity was 98% (95% CrI 97% to 99%) for smear-positive, culture-positive TB and 68% (95% CrI 59% to 75%) for smear-negative, culture-positive TB (15 studies); the pooled sensitivity was 80% (95% CrI 67% to 88%) in people living with HIV and 89% (95% CrI 81% to 94%) in people without HIV infection (four studies). For rifampicin resistance detection (11 studies, 2340 participants), Xpert achieved a pooled sensitivity of 94% (95% CrI 87% to 97%) and pooled specificity of 98% (95% CrI 97% to 99%). In a separate analysis, Xpert could distinguish between TB and nontuberculous mycobacteria (NTM) in clinical samples with high accuracy: among 139 specimens with NTM, Xpert was positive in only one specimen that grew NTM.In a hypothetical cohort of 1000 individuals suspected of having rifampicin resistance (a proxy for MDR-TB), where the prevalence of rifampicin resistance is 30%, we estimated that on average Xpert would wrongly identify 14 patients as being rifampicin resistant. In comparison, where the prevalence of rifampicin resistance is only 2%, we estimated that the number of individuals wrongly identified as rifampicin resistant would increase to 20, an increase of 43%.
Authors' conclusions: This review shows that Xpert used as an initial diagnostic test for TB detection and rifampicin resistance detection in patients suspected of having TB, MDR-TB, or HIV-associated TB is sensitive and specific. Xpert may also be valuable as an add-on test following microscopy for patients who have previously been found to be smear-negative. An Xpert result that is positive for rifampicin resistance should be carefully interpreted and take into consideration the risk of MDR-TB in a given patient and the expected prevalence of MDR-TB in a given setting.Studies in this review mainly assessed sensitivity and specificity of the test when used in reference laboratories in research investigations. Most studies were performed in high TB burden countries. Ongoing use of Xpert in high TB burden countries will contribute to the evidence base on the diagnostic accuracy and clinical impact of Xpert in routine programmatic and peripheral health care settings, including settings where the test is performed at the point of care.
Figures
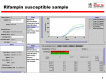
Figure 1
Readout of Xpert MTB/RIF assay for a TB positive, rifampicin-susceptible specimen. Courtesy: Karin Weyer, The WHO STOP TB Department.
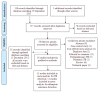
Figure 2
Flow diagram of studies in the review.
Figure 3
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across the 27 included study centres (18 studies). The reference standard domain pertains to TB as the target condition. See text for the reference standard pertaining to rifampicin resistance.
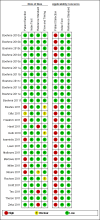
Figure 4
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study centre.
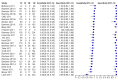
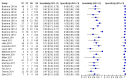
Figure 5
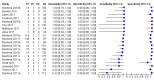
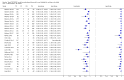
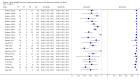
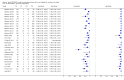
Forest plots of Xpert sensitivity and specificity for TB detection, Xpert used as an initial test replacing smear microscopy. The individual studies are ordered by decreasing sensitivity. TP = True Positive; FP = False Positive; FN = False Negative; TN = True Negative. Between brackets are the 95% CI of sensitivity and specificity. The figure shows the estimated sensitivity and specificity of the study (blue square) and its 95% CI (black horizontal line). Xpert specificity could not be estimated in one study.
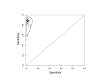
Figure 6
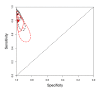
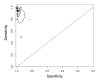
Summary plots of Xpert sensitivity and specificity for TB detection, Xpert used as an initial test replacing smear microscopy. Each individual study is represented by an empty square. The size of the square is proportional to the sample size of the study such that larger studies are represented by larger squares. The filled circle is the pooled median estimate for sensitivity and specificity. The solid curves represent the 95% credible region around the summary estimate; the dashed curves represent the 95% prediction region.
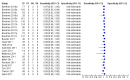
Figure 7
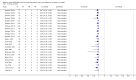
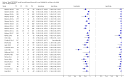
Forest plots of Xpert for TB detection, Xpert used as an add-on test following a negative smear microscopy result. TP = True Positive; FP = False Positive; FN = False Negative; TN = True Negative. Between brackets the 95% CI of sensitivity and specificity. The figure shows the estimated sensitivity and specificity of the study (blue square) and its 95% CI (black horizontal line).
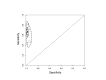
Figure 8
Summary plots of Xpert sensitivity and specificity for TB detection, Xpert used as an add-on test following a negative smear microscopy result. Each individual study is represented by an empty square. The size of the square is proportional to the sample size of the study such that larger studies are represented by larger squares. The filled circle is the pooled median estimate for sensitivity and specificity. The solid curve represents the 95% credible region around the summary estimate; the dashed curves represent the 95% prediction region.
Figure 9
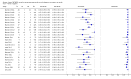
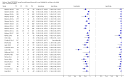
Forest plot of Xpert sensitivity for TB detection in smear-positive subgroup. The squares represent the sensitivity and specificity of one study, the black line its CI. TP = true positive; FP = false positive; FN = false negative; TN = true negative. Xpert specificity could not be estimated in these studies.
Figure 10
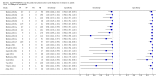
Forest plots of Xpert sensitivity and specificity for TB detection in HIV-positive and HIV-negative subgroups. The squares represent the sensitivity and specificity of one study and the black line represent its CI. TP = true positive; FP = false positive; FN = false negative; TN = true negative.
Figure 11
Summary plots of Xpert sensitivity and specificity for TB detection in HIV-positive (red colour) and HIV-negative subgroups (black colour). Each individual study is represented by an empty square. The size of the square is proportional to the sample size of the study such that larger studies are represented by larger squares. The filled circles are the median estimates for sensitivity and specificity. The solid curves represent the 95% credible region around the summary estimates; the dashed curves represent the 95% prediction region.
Figure 12
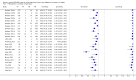
Forest plots of Xpert sensitivity and specificity for detection of rifampicin resistance, Xpert used as an initial test replacing conventional drug susceptibility testing as the initial test. The individual studies are ordered by decreasing sensitivity and decreasing number of true positives. The squares represent the sensitivity and specificity of one study, the black line its CI. TP = true positive; FP = false positive; FN = false negative; TN = true negative.
Figure 13
Summary plots of Xpert sensitivity and specificity for detection of rifampicin resistance, Xpert used as an initial test replacing conventional drug susceptibility testing as the initial test. Each individual study is represented by an empty square. The size of the square is proportional to the sample size of the study such that larger studies are represented by larger squares. The filled circle is the pooled median estimate for sensitivity and specificity. The solid curves represent the 95% credible region around the summary estimate; the dashed curves represent the 95% prediction region.
Figure 14
Bayesian bivariate hierarchical model, likelihood
Figure 15
Bayesian bivariate hierarchical model, prior distributions
Test 1.
TB detection, all studies.
Test 2.
Add on.
Test 3.
Smear positive.
Test 4.
Smear negative.
Test 5.
HIV positive.
Test 6.
HIV negative.
Test 7.
TB detection, condition of specimen.
Test 8.
TB detection, specimen preparation.
Test 9.
TB prevalence.
Test 10.
Income status.
Test 11.
Rifampicin resistance.
Test 12.
RIF resistance prevalence.
Comment in
- In reply to 'False-positive Xpert® MTB/RIF assays in previously treated patients'.
Steingart KR, Schiller I, Dendukuri N. Steingart KR, et al. Int J Tuberc Lung Dis. 2015 Mar;19(3):366-7. doi: 10.5588/ijtld.14.0800. Int J Tuberc Lung Dis. 2015. PMID: 25686149 No abstract available.
Similar articles
- Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults.
Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Steingart KR, et al. Cochrane Database Syst Rev. 2014 Jan 21;2014(1):CD009593. doi: 10.1002/14651858.CD009593.pub3. Cochrane Database Syst Rev. 2014. PMID: 24448973 Free PMC article. Updated. Review. - Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance.
Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, Steingart KR. Kohli M, et al. Cochrane Database Syst Rev. 2018 Aug 27;8(8):CD012768. doi: 10.1002/14651858.CD012768.pub2. Cochrane Database Syst Rev. 2018. PMID: 30148542 Free PMC article. Updated. Review. - Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis.
Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, Ochodo EA, Haraka F, Zwerling AA, Pai M, Steingart KR, Horne DJ. Zifodya JS, et al. Cochrane Database Syst Rev. 2021 Feb 22;2:CD009593. doi: 10.1002/14651858.CD009593.pub5. Cochrane Database Syst Rev. 2021. PMID: 33616229 - Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children.
Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Detjen AK, Steingart KR, Mandalakas AM. Kay AW, et al. Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD013359. doi: 10.1002/14651858.CD013359.pub2. Cochrane Database Syst Rev. 2020. PMID: 32853411 Free PMC article. Updated. - Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms.
Shapiro AE, Ross JM, Yao M, Schiller I, Kohli M, Dendukuri N, Steingart KR, Horne DJ. Shapiro AE, et al. Cochrane Database Syst Rev. 2021 Mar 23;3(3):CD013694. doi: 10.1002/14651858.CD013694.pub2. Cochrane Database Syst Rev. 2021. PMID: 33755189 Free PMC article.
Cited by
- Presentation and outcome of COVID-19 in HIV patients with high viral loads and opportunistic infections: a case series.
Hardy YO, Amenuke DA, Hutton-Mensah KA, Chadwick DR, Larsen-Reindorf R. Hardy YO, et al. Ghana Med J. 2020 Dec;54(4 Suppl):121-124. doi: 10.4314/gmj.v54i4s.20. Ghana Med J. 2020. PMID: 33976453 Free PMC article. - Evidence supports TB test, so what now?
Cohen D, Corbett E. Cohen D, et al. Cochrane Database Syst Rev. 2013 Jan 31;2013(2):ED000051. doi: 10.1002/14651858.ED000051. Cochrane Database Syst Rev. 2013. PMID: 23450616 Free PMC article. No abstract available. - Implementation of Xpert MTB/RIF in Uganda: Missed Opportunities to Improve Diagnosis of Tuberculosis.
Hanrahan CF, Haguma P, Ochom E, Kinera I, Cobelens F, Cattamanchi A, Davis L, Katamba A, Dowdy D. Hanrahan CF, et al. Open Forum Infect Dis. 2016 May 12;3(2):ofw068. doi: 10.1093/ofid/ofw068. eCollection 2016 Mar. Open Forum Infect Dis. 2016. PMID: 27186589 Free PMC article. - Recent advances in tuberculosis and nontuberculous mycobacteria lung disease.
Park JS. Park JS. Tuberc Respir Dis (Seoul). 2013 Jun;74(6):251-5. doi: 10.4046/trd.2013.74.6.251. Epub 2013 Jun 25. Tuberc Respir Dis (Seoul). 2013. PMID: 23814596 Free PMC article. - Outbreak of Tuberculosis and Multidrug-Resistant Tuberculosis, Mbuji-Mayi Central Prison, Democratic Republic of the Congo.
Kayomo MK, Hasker E, Aloni M, Nkuku L, Kazadi M, Kabengele T, Muteteke D, Kapita F, Lufulwabo A, Mukadi YD, Muyembe-Tamfum JJ, Ieven M, de Jong BC, Boelaert M. Kayomo MK, et al. Emerg Infect Dis. 2018 Nov;24(11):2029-2035. doi: 10.3201/eid2411.180769. Emerg Infect Dis. 2018. PMID: 30334730 Free PMC article.
References
References to studies excluded from this review
- Armand S, Vanhuls P, Delcroix G, Courcol R, Lemaitre N. Comparison of the Xpert MTB/RIF test with an IS6110-TaqMan real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. Journal of Clinical Microbiology. 2011;49((5)):1772–6. - PMC - PubMed
References to ongoing studies
- Multicentre randomised control trial of point-of-treatment (Clinic-based) Xpert MTB/RIF assay. Ongoing study 7 July 2011.
- Evaluation of Xpert MTB/RIF assay for the rapid identification of TB and TB rifampin resistance in HIV-infected and HIV-uninfected pulmonary tuberculosis suspects. Ongoing study 24 April 2012.
- A randomised control trial of sputum induction, and new and emerging technologies in a high HIV prevalence primary care setting. Ongoing study August 2009.
Additional references
- Abimbola TO, Marston BJ, Date AA, Blandford JM, Sangrujee N, Wiktor SZ. Cost-effectiveness of tuberculosis diagnostic strategies to reduce early mortality among persons with advanced HIV infection initiating antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2012;60((1)):e1–7. - PubMed
- Boehme CC, Nabeta P, Henostroza G, Raqib R, Rahim Z, Gerhardt M, et al. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. Journal of Clinical Microbiology. 2007;45((6)):1936–40. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials