Arginase inhibition alleviates hypertension in the metabolic syndrome - PubMed (original) (raw)
Comparative Study
. 2013 Jun;169(3):693-703.
doi: 10.1111/bph.12144.
Affiliations
- PMID: 23441715
- PMCID: PMC3682715
- DOI: 10.1111/bph.12144
Comparative Study
Arginase inhibition alleviates hypertension in the metabolic syndrome
Hany M El-Bassossy et al. Br J Pharmacol. 2013 Jun.
Abstract
Background and purpose: We have previously shown that arginase inhibition alleviates hypertension associated with in a diabetic animal model. Here, we investigated the protective effect of arginase inhibition on hypertension in metabolic syndrome.
Experimental approach: Metabolic syndrome was induced in rats by administration of fructose (10% in drinking water) for 12 weeks to induce vascular dysfunction. Three arginase inhibitors (citrulline, norvaline and ornithine) were administered daily in the last 6 weeks of study before and tail BP was recorded in conscious animals. Concentration response curves for phenylephrine (PE), KCl and ACh in addition to ACh-induced NO generation were obtained in thoracic aorta rings. Serum glucose, insulin, uric acid and lipid profile were determined as well as reactive oxygen species (ROS) and arginase activity.
Key results: Arginase activity was elevated in metabolic syndrome while significantly inhibited by citrulline, norvaline or ornithine treatment. Metabolic syndrome was associated with elevations in systolic and diastolic BP, while arginase inhibition significantly reduced elevations in diastolic and systolic BP. Metabolic syndrome increased vasoconstriction responses of aorta to PE and KCl and decreased vasorelaxation to ACh, while arginase inhibition completely prevented impaired responses to ACh. In addition, arginase inhibition prevented impaired NO generation and exaggerated ROS formation in metabolic syndrome. Furthermore, arginase inhibition significantly reduced hyperinsulinaemia and hypertriglyceridaemia without affecting hyperuricaemia or hypercholesterolaemia associated with metabolic syndrome.
Conclusions and implications: Arginase inhibition alleviates hypertension in metabolic syndrome directly through endothelial-dependent relaxation/NO signalling protection and indirectly through inhibition of insulin resistance and hypertriglyceridaemia.
© 2013 The Authors. British Journal of Pharmacology © 2013 The British Pharmacological Society.
Figures
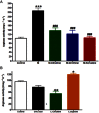
Figure 1
Effect of fructose-induced metabolic syndrome (M, 10% in drinking water, for 12 weeks) and daily oral administration (last 6 weeks) of citrulline (50 mg·kg−1), norvaline (50 mg·kg−1) or ornithine (200 mg·kg−1) on serum arginase activity (A) or the effect of in vitro incubation with uric acid (400 μM, 1 h), citrulline (1 mM, 1 h) or arginine (1 mM, 1 h) on aortic arginase activity (B). *P < 0.05, **P < 0.01, ***P < 0.001, compared with the corresponding control group values; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the corresponding M group values; by one-way
anova
and Newman–Keuls post hoc test.
Figure 2
Effect of fructose- induced metabolic syndrome (M, 10% in drinking water, for 12 weeks) and daily oral administration (last 6 weeks) of citrulline (50 mg·kg−1), norvaline (50 mg·kg−1) or ornithine (200 mg·kg−1) on the systolic (A) and diastolic (B) blood pressure. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the corresponding control group values; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the corresponding M group values; by one-way
anova
and Newman–Keuls post hoc test.
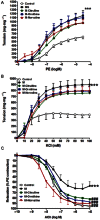
Figure 3
Effect of fructose- induced metabolic syndrome (M, 10% in drinking water, for 12 weeks) and daily oral administration (last 6 weeks) of citrulline (50 mg·kg−1), norvaline (50 mg·kg−1) or ornithine (200 mg·kg−1) on the isolated aorta responsiveness to PE (A), KCl (B) and ACh (C). Symbols indicate mean ± SEM for n = 6–8 animals; *P < 0.05, **P < 0.01, ***P < 0.001, compared with the corresponding control group values; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the corresponding M group values; by one-way
anova
and Newman–Keuls post hoc test.
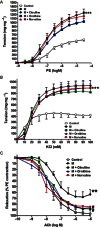
Figure 4
Effect of in vitro incubation with citrulline (1 mM, 1 h), norvaline (1 mM, 1 h) or ornithine (1 mM, 1 h) on the isolated metabolic syndrome (M) aorta responsiveness to PE (A), KCl (B) and ACh (C). Symbols indicate mean ± SEM for n = 6–8 animals; *P < 0.05, **P < 0.01, ***P < 0.001, compared with the corresponding control group values; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the corresponding M group values; by one-way
anova
and Newman–Keuls post hoc test.
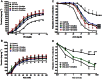
Figure 5
Effect of in vitro incubation with uric acid (200 μM, 1 h) with or without cirulline (1 mM, 1 h), norvaline (1 mM, 1 h) or ornithine (1 mM, 1 h) on the isolated normal aorta responsiveness to PE (A), KCl (B) and ACh (C) or on the isolated perfused kidney relaxation to ACh (D). Symbols indicate mean ± SEM for n = 6–8 animals; *P < 0.05, **P < 0.01, ***P < 0.001, compared with the corresponding control group values; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the corresponding uric acid values; by one-way
anova
and Newman–Keuls post hoc test.
Figure 6
Effect of fructose- induced metabolic syndrome (M, 10% in drinking water, for 12 weeks) and chronic daily oral administration (last 6 weeks) of citrulline (50 mg·kg−1), norvaline (50 mg·kg−1) or ornithine (200 mg·kg−1) on ACh-stimulated NO generation (A) and basal ROS formation (B). Symbols indicate mean ± SEM for n = 6–8 animals; *P < 0.05, **P < 0.01, ***P < 0.001, compared with the corresponding control group values; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the corresponding M group values; by one-way
anova
and Newman–Keuls post hoc test.
Similar articles
- Arginase inhibition alleviates hypertension associated with diabetes: effect on endothelial dependent relaxation and NO production.
El-Bassossy HM, El-Fawal R, Fahmy A. El-Bassossy HM, et al. Vascul Pharmacol. 2012 Nov-Dec;57(5-6):194-200. doi: 10.1016/j.vph.2012.01.001. Epub 2012 Jan 17. Vascul Pharmacol. 2012. PMID: 22281732 - Characterization of vascular complications in experimental model of fructose-induced metabolic syndrome.
El-Bassossy HM, Dsokey N, Fahmy A. El-Bassossy HM, et al. Toxicol Mech Methods. 2014 Dec;24(8):536-43. doi: 10.3109/15376516.2014.945109. Epub 2014 Jul 29. Toxicol Mech Methods. 2014. PMID: 25046175 - Heme oxygenase-1 alleviates vascular complications associated with metabolic syndrome: Effect on endothelial dependent relaxation and NO production.
El-Bassossy HM, Hassan N, Zakaria MN. El-Bassossy HM, et al. Chem Biol Interact. 2014 Nov 5;223:109-15. doi: 10.1016/j.cbi.2014.09.014. Epub 2014 Sep 28. Chem Biol Interact. 2014. PMID: 25268984 - l-Citrulline Supplementation: Impact on Cardiometabolic Health.
Allerton TD, Proctor DN, Stephens JM, Dugas TR, Spielmann G, Irving BA. Allerton TD, et al. Nutrients. 2018 Jul 19;10(7):921. doi: 10.3390/nu10070921. Nutrients. 2018. PMID: 30029482 Free PMC article. Review. - Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal?
Pernow J, Jung C. Pernow J, et al. Cardiovasc Res. 2013 Jun 1;98(3):334-43. doi: 10.1093/cvr/cvt036. Epub 2013 Feb 14. Cardiovasc Res. 2013. PMID: 23417041 Review.
Cited by
- Neonatal Citrulline Supplementation and Later Exposure to a High Fructose Diet in Rats Born with a Low Birth Weight: A Preliminary Report.
Tran NT, Alexandre-Gouabau MC, Pagniez A, Ouguerram K, Boquien CY, Winer N, Darmaun D. Tran NT, et al. Nutrients. 2017 Apr 11;9(4):375. doi: 10.3390/nu9040375. Nutrients. 2017. PMID: 28398243 Free PMC article. - L-Norvaline Reverses Cognitive Decline and Synaptic Loss in a Murine Model of Alzheimer's Disease.
Polis B, Srikanth KD, Elliott E, Gil-Henn H, Samson AO. Polis B, et al. Neurotherapeutics. 2018 Oct;15(4):1036-1054. doi: 10.1007/s13311-018-0669-5. Neurotherapeutics. 2018. PMID: 30288668 Free PMC article. - Antiglycation Activities and Common Mechanisms Mediating Vasculoprotective Effect of Quercetin and Chrysin in Metabolic Syndrome.
Ahmed OAA, Azhar AS, Tarkhan MM, Balamash KS, El-Bassossy HM. Ahmed OAA, et al. Evid Based Complement Alternat Med. 2020 Jul 27;2020:3439624. doi: 10.1155/2020/3439624. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32802123 Free PMC article. - Asymmetric dimethylarginine (ADMA) elevation and arginase up-regulation contribute to endothelial dysfunction related to insulin resistance in rats and morbidly obese humans.
El Assar M, Angulo J, Santos-Ruiz M, Ruiz de Adana JC, Pindado ML, Sánchez-Ferrer A, Hernández A, Rodríguez-Mañas L. El Assar M, et al. J Physiol. 2016 Jun 1;594(11):3045-60. doi: 10.1113/JP271836. Epub 2016 Mar 4. J Physiol. 2016. PMID: 26840628 Free PMC article.
References
- Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. - PubMed
- Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. - PubMed
- Cederbaum SD, Yu H, Grody WW, Kern RM, Yoo P, Iyer RK. Arginases I and II: do their functions overlap? Mol Genet Metab. 2004;81(Suppl. 1):S38–S44. - PubMed
- El-Bassossy HM, El-Moselhy MA, Mahmoud MF. Pentoxifylline alleviates vascular impairment in insulin resistance via TNF-α inhibition. Naunyn Schmiedebergs Arch Pharmacol. 2011a;384:277–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical