Switching from single-stranded to double-stranded DNA limits the unwinding processivity of ring-shaped T7 DNA helicase - PubMed (original) (raw)
Switching from single-stranded to double-stranded DNA limits the unwinding processivity of ring-shaped T7 DNA helicase
Yong-Joo Jeong et al. Nucleic Acids Res. 2013 Apr.
Abstract
Phage T7 helicase unwinds double-stranded DNA (dsDNA) by encircling one strand while excluding the complementary strand from its central channel. When T7 helicase translocates on single-stranded DNA (ssDNA), it has kilobase processivity; yet, it is unable to processively unwind linear dsDNA, even 60 base-pairs long. Particularly, the GC-rich dsDNAs are unwound with lower amplitudes under single-turnover conditions. Here, we provide evidence that T7 helicase switches from ssDNA to dsDNA during DNA unwinding. The switching propensity is higher when dsDNA is GC-rich or when the 3'-overhang of forked DNA is <15 bases. Once helicase encircles dsDNA, it travels along dsDNA and dissociates from the end of linear DNA without strand separation, which explains the low unwinding amplitude of these substrates. Trapping the displaced strand with ssDNA binding protein or changing its composition to morpholino oligomer that does not interact with helicase increases the unwinding amplitude. We conclude that the displaced strand must be continuously excluded and kept away from the central channel for processive DNA unwinding. The finding that T7 helicase can switch from ssDNA to dsDNA binding mode during unwinding provides new insights into ways of limiting DNA unwinding and triggering fork regression when stalled forks need to be restarted.
Figures
Figure 1.
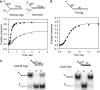
DNA unwinding analysis of fork DNAs with modified 3′-overhangs. The single turnover unwinding kinetics was measured at 18°C as described in the Experimental Procedures. (A) shows the unwinding kinetics of ds30-5T (filled triangle), ds30-10T (filled circle) and ds30-15T (open square) fit to Equations (2)–(4) (Experimental Procedures) to obtain the average unwinding rate (_k_u = k × s) and amplitude (A) for ds30-10T, _k_u = 33 bp/s (A = 0.68) and ds30-15T, _k_u = 26 bp/s (A = 0.91). (B) Native polyacrylamide gel image shows the unwinding products of tandem substrate under single turnover reaction conditions. Left panel, lanes 1–3 are standards, and lane 4 shows products after 2 min of reaction. Middle panel, lane 1 shows reaction after 2 min, and lanes 2–4 are standards. Right panel, lane 1 shows reaction after 2 min, and lanes 2–4 are standards. (C) The unwinding kinetics of ds30-15T (filled circle) in comparison with that of the ds30-B·S (filled triangle) and ds30-hp (open square) DNAs. The average unwinding rates for ds30-B·S, _k_u = 19 bp/s (A = 0.8), ds30-hp, _k_u = 18 bp/s (A = 0.78). Asterisk represents the position of radiolabelling.
Figure 2.
Unwinding kinetics of 3′-morpholino/5′-DNA forked substrate by T7 helicase. (A) Single-turnover unwinding kinetics of ds18-DNA:DNA (open circle) and ds18-DNA:M-oligo (filled circle) with 7 nt 3′-tail fit to average unwinding rates _k_u = 5 bp/s (A = 0.56) and _k_u = 35 bp/s (A = 0.9), respectively. (B) The ds25-DNA:M-oligo without any 3′-tail was unwound with single-turnover rate, _k_u = 26 bp/s (A = 0.52). (C) The native polyacrylamide gel image shows unwinding of the ds25-DNA:M-oligo (left panel) and ds25-DNA:DNA (right panel) under single-turnover conditions. Lanes 1 and 4 show the control unreacted starting DNA. Lanes 2 and 5 show the control radiolabelled 5′-strand. Lanes 3 and 6 show the reaction products after 1 min of unwinding.
Figure 3.
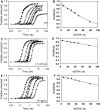
Unwinding kinetics in the presence and absence of ssDNA-binding proteins. The single-turnover unwinding kinetics was measured for ds18 (Filled circle), ds30 (○), ds40 (filled inverted triangle), ds50 (open inverted triangle), ds60 (filled square) and ds90 (open square) at 18°C in the absence of ssDNA-binding protein (A), in the presence of E. coli SSB (C) or in the presence of T7 gp2.5 (E). Each curve was fit to Equations (2)–(4) to obtain the unwinding rates. The average unwinding rate is 19 ± 7 bp/s in the absence of ssDNA-binding protein, 32 ± 5 bp/s in the presence of E. coli SSB and 23 ± 8 bp/s in the presence of T7 gp2.5. The amplitude from the individual fits is plotted against the corrected dsDNA length (_L-L_m) and fit to Equation (5) to obtain processivity of 0.9911 (±0.0007) in the absence of ssDNA-binding proteins (B), 0.9979 (±0.0002) in the presence of E. coli SSB (D) and 0.9971 (±0.0003) in the presence of T7 gp2.5 (F).
Figure 4.
Unwinding kinetics of GC-rich forked and tandem substrate. (A) Single turnover unwinding kinetics of the 30 bp 100% GC dsDNA with and without E. coli SSB. The average unwinding rate was _k_u = 8 bp/s (A = 0.046) in the absence of SSB and _k_u = 10 bp/s (A = 0.44) in the presence of E. coli SSB. (B) The proximal 100% GC-rich dsDNA region of the tandem forked DNA substrate was unwound with _k_u = 6 bp/s (A = 0.13) and the distal 50% GC rich dsDNA region was unwound with _k_u = 5.2 bp/s (A = 0.46). (C) The schematic representation shows the normal pathway of unwinding both dsDNA regions of the tandem dsDNA substrate, and the pathway where the helicase encircles and does not unwind the initial GC-rich dsDNA but unwinds the distal dsDNA region.
Figure 5.
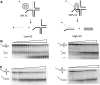
Unwinding and branch migration kinetics on GC-rich forked four-way junction. (A) Schematic representation of the unwinding of forked four-way junction substrates by T7 helicase. The Low-GC substrate (left panel) is preferentially unwound by T7 helicase, whereas the high-GC substrates (right panel) is preferentially resolved. (B) Single-turnover unwinding kinetics (0.1–120 s) of forked four-way junction substrates with a low-GC loading arm (left panel) or a high-GC loading arm (right panel). The low-GC substrate gets unwound, whereas the high-GC substrate gets resolved. (C) Single-turnover unwinding kinetics (0.1–120 s) of control four-way junction substrates lacking the 3′-overhang get resolved irrespective of the GC-content.
Figure 6.
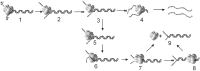
Model that explains the low processivity of DNA unwinding by T7 helicase. T7 helicase initiates unwinding from the 5′-ssDNA overhang. The upper highlighted pathway (species 1–4) shows a series of intermediates that result in the complete unwinding of the dsDNA. The branched pathway (3–9) shows that the occasional ring opening reaction (3→4→5) can result in T7 helicase encircling the displaced strand and the dsDNA (5→6→7). The helicase ring bound to the dsDNA can dissociate in solution (7→9) or move along the dsDNA (7→8) and fall off from the end of the linear DNA without unwinding the DNA strands (8→9).
Similar articles
- Asymmetric interactions of hexameric bacteriophage T7 DNA helicase with the 5'- and 3'-tails of the forked DNA substrate.
Ahnert P, Patel SS. Ahnert P, et al. J Biol Chem. 1997 Dec 19;272(51):32267-73. doi: 10.1074/jbc.272.51.32267. J Biol Chem. 1997. PMID: 9405431 - ATP-induced helicase slippage reveals highly coordinated subunits.
Sun B, Johnson DS, Patel G, Smith BY, Pandey M, Patel SS, Wang MD. Sun B, et al. Nature. 2011 Sep 18;478(7367):132-5. doi: 10.1038/nature10409. Nature. 2011. PMID: 21927003 Free PMC article. - T7 DNA helicase: a molecular motor that processively and unidirectionally translocates along single-stranded DNA.
Kim DE, Narayan M, Patel SS. Kim DE, et al. J Mol Biol. 2002 Aug 30;321(5):807-19. doi: 10.1016/s0022-2836(02)00733-7. J Mol Biol. 2002. PMID: 12206763 - Mechanisms of a ring shaped helicase.
Donmez I, Patel SS. Donmez I, et al. Nucleic Acids Res. 2006;34(15):4216-24. doi: 10.1093/nar/gkl508. Epub 2006 Aug 25. Nucleic Acids Res. 2006. PMID: 16935879 Free PMC article. Review. - Dynamic coupling between the motors of DNA replication: hexameric helicase, DNA polymerase, and primase.
Patel SS, Pandey M, Nandakumar D. Patel SS, et al. Curr Opin Chem Biol. 2011 Oct;15(5):595-605. doi: 10.1016/j.cbpa.2011.08.003. Epub 2011 Aug 22. Curr Opin Chem Biol. 2011. PMID: 21865075 Free PMC article. Review.
Cited by
- Old, new, and widely true: The bacteriophage T4 DNA packaging mechanism.
Black LW. Black LW. Virology. 2015 May;479-480:650-6. doi: 10.1016/j.virol.2015.01.015. Epub 2015 Feb 27. Virology. 2015. PMID: 25728298 Free PMC article. Review. - The DNA translocase FANCM/MHF promotes replication traverse of DNA interstrand crosslinks.
Huang J, Liu S, Bellani MA, Thazhathveetil AK, Ling C, de Winter JP, Wang Y, Wang W, Seidman MM. Huang J, et al. Mol Cell. 2013 Nov 7;52(3):434-46. doi: 10.1016/j.molcel.2013.09.021. Epub 2013 Oct 24. Mol Cell. 2013. PMID: 24207054 Free PMC article. - Mechanism of eukaryotic origin unwinding is a dual helicase DNA shearing process.
Langston LD, Georgescu RE, O'Donnell ME. Langston LD, et al. Proc Natl Acad Sci U S A. 2023 Dec 26;120(52):e2316466120. doi: 10.1073/pnas.2316466120. Epub 2023 Dec 18. Proc Natl Acad Sci U S A. 2023. PMID: 38109526 Free PMC article. - Targeted chromosomal Escherichia coli:dnaB exterior surface residues regulate DNA helicase behavior to maintain genomic stability and organismal fitness.
Behrmann MS, Perera HM, Hoang JM, Venkat TA, Visser BJ, Bates D, Trakselis MA. Behrmann MS, et al. PLoS Genet. 2021 Nov 12;17(11):e1009886. doi: 10.1371/journal.pgen.1009886. eCollection 2021 Nov. PLoS Genet. 2021. PMID: 34767550 Free PMC article. - Single-molecule imaging of FtsK translocation reveals mechanistic features of protein-protein collisions on DNA.
Lee JY, Finkelstein IJ, Arciszewska LK, Sherratt DJ, Greene EC. Lee JY, et al. Mol Cell. 2014 Jun 5;54(5):832-43. doi: 10.1016/j.molcel.2014.03.033. Epub 2014 Apr 24. Mol Cell. 2014. PMID: 24768536 Free PMC article.
References
- Matson SW, Bean DW, George JW. DNA helicases: enzymes with essential roles in all aspects of DNA metabolism. BioEssays. 1994;16:13–22. - PubMed
- Lohman TM, Bjornson KP. Mechanisms of helicase-catalyzed DNA unwinding. Annu. Rev. Biochem. 1996;65:169–214. - PubMed
- Levin MK, Patel SS. In: Molecular Motors. Schliwa M, editor. Weinheim Germany: Wiley-VCH Verlag GmbH; 2003. pp. 179–198.
- Patel SS, Picha KM. Structure and function of hexameric helicases. Annu. Rev. Biochem. 2000;69:651–697. - PubMed
- Patel SS, Donmez I. Mechanisms of helicases. J. Biol. Chem. 2006;281:18265–18268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous