Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma - PubMed (original) (raw)
Comment
Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma
Cindy Barnig et al. Sci Transl Med. 2013.
Abstract
Asthma is a prevalent disease of chronic inflammation in which endogenous counterregulatory signaling pathways are dysregulated. Recent evidence suggests that innate lymphoid cells (ILCs), including natural killer (NK) cells and type 2 ILCs (ILC2s), can participate in the regulation of allergic airway responses, in particular airway mucosal inflammation. We have identified both NK cells and ILC2s in human lung and peripheral blood in healthy and asthmatic subjects. NK cells were highly activated in severe asthma, were linked to eosinophilia, and interacted with autologous eosinophils to promote their apoptosis. ILC2s generated antigen-independent interleukin-13 (IL-13) in response to the mast cell product prostaglandin D2 alone and in a synergistic manner with the airway epithelial cytokines IL-25 and IL-33. Both NK cells and ILC2s expressed the pro-resolving ALX/FPR2 receptors. Lipoxin A4, a natural pro-resolving ligand for ALX/FPR2 receptors, significantly increased NK cell-mediated eosinophil apoptosis and decreased IL-13 release by ILC2s. Together, these findings indicate that ILCs are targets for lipoxin A4 to decrease airway inflammation and mediate the catabasis of eosinophilic inflammation. Because lipoxin A4 generation is decreased in severe asthma, these findings also implicate unrestrained ILC activation in asthma pathobiology.
Conflict of interest statement
Competing interest:
BDL is a co-inventor on patents related to lipoxin A4 and asthma that have been licensed by the Brigham and Women’s Hospital (BWH) for clinical development and he receives a share of licensing income through BWH. None of the other authors declare any financial competing interests.
Figures
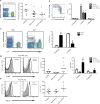
Fig. 1. NK cells are activated in asthma
Peripheral venous blood was obtained from healthy subjects and individuals with mild and severe asthma. (A) NK cells were identified as a NKp46+CD3− cell population in a lymphocyte morphology gate based on SSC and FSC characteristics (violet box, left panel) and NK cells as a percentage of total lymphocytes were enumerated (right panel), bar indicates mean value. (B) NK cells were further phenotyped by flow cytometry analysis of CD56 expression for CD56bright and CD56dim subsets (violet boxes, left panel) in samples from healthy and asthmatic subjects (right panel). (C) Paired samples of BALF and blood were obtained on a subset of severe asthmatics. Representative FACS plots show an enriched NKp46bright cell population in BALF compared to PBMCs (left panel). BALFs were also enriched for CD56bright subset of NK cells (n=5) (right panel). (D) Representative FACS histograms showing CD69 expression in peripheral blood NK cells (left panel) that was upregulated in severe asthma (middle panel), in particular in the CD56dim subset (right panel). (E) Representative FACS histograms showing NKG2D expression in peripheral NK cells (left panel) that was upregulated in severe asthma (right panel). Values are expressed as mean ± s.e.m.; *, P < 0.05, compared with healthy subjects (one-way ANOVA); §, P < 0.0001, compared with PBMCs (one-way ANOVA); **, P < 0.05, compared with healthy and mild asthmatic subjects (one-way ANOVA).
Fig. 2. NK cells interact with eosinophils
(A–C) The percentage of peripheral blood eosinophils in severe asthma was correlated to (A) the percentage of peripheral blood NK cells; Spearman r=0.86, P < 0.05. (B) NK cell CD69 expression; Pearson r=0.50, P < 0.05, and (C) NK cell NKG2D expression, Spearman r=0.59, P < 0.01. (D–F) Incubation (4h, 37°C) of autologous peripheral blood granulocytes with NK cells (1:5 ratio) triggered increased granulocyte apoptosis compared to granulocytes incubated alone by (D) morphological appearance on cytospin (arrows, apoptotic granulocytes) and (E,F) by FACS criteria (Annexin V+7-AAD−) for (E) eosinophils (Eos) and (F) neutrophils (PMN) in samples of peripheral blood from healthy subjects and individuals with mild or severe asthma. Results are expressed as mean ± SEM.; n=5 individual healthy donors, n=5 mild asthmatic donors, n=5 severe asthmatic donors; *, P < 0.05, compared with granulocytes alone and **, P < 0.05 compared with healthy donors (Student’s _t_-test).
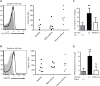
Fig. 3. NK cells express pro-resolving receptors and respond to LXA4
(A) Flow cytometry histograms for anti-ALX (FPRL1) Ab (white) and isotype control (gray) for peripheral blood NK cells (left panel) and MFI for ALX expression on peripheral NK cells in healthy subjects and mild and severe asthmatic subjects (right panel). (B) Flow cytometry histograms for anti-CMKLR1 Ab (white) and isotype control (gray) for peripheral blood NK cells (left panel) and MFI for CMKLR1 expression on peripheral NK cells in healthy subjects and mild and severe asthmatic subjects (right panel). *, P < 0.05, compared with healthy and mild asthmatic subjects (one-way ANOVA). (C, D) Autologous granulocytes and NK cells from healthy subjects were selectively exposed to lipoxin A4 (LX, 100 nM, 15 min, 37°C) in the absence or presence of the ALX/FPR2 antagonist (WRW4, 230nM) prior to co-incubation and the percent change in apoptosis was determined for (C) eosinophils (Eos) and (D) neutrophils (PMN) (see Methods). Results are expressed as mean ± SEM; n≥4; **, P < 0.05, compared with control (one-way ANOVA); §, P < 0.05, compared with NK(LX).
Fig. 4. ILC2 are present in peripheral blood of healthy and asthmatic subjects and express pro-resolving receptors
(A–D) Flow cytometry gating strategy for the identification of helper ILC and the ILC2 subset. PBMCs were depleted of most T and B lymphocytes and monocytes, and a lymphoid morphology cell population was selected based on their forward and side scatter characteristics. (A) Flow cytometry analysis showing the presence of a Lin− cell population (violet box, CD3−TCRαβ−TCRγδ−CD19−CD14−CD16−CD34−CD123−CD11c−) different from NK cells (Lin−CD56+), left panel; identification of a Lin−CD127+CD117+/− population, right panel. (B) Lin−CD127+CD117+/− expressed NK cell receptors, including CD161, left panel; NKG2D, right panel, and (C) NKp46. (D) ILC2 were identified as a subpopulation of Lin−CD127+ cells that also expressed CRTH2. (E) The number of Lin−CD127+CRTH2+ ILC2 cells was determined in healthy and mild and severe asthmatic subjects. (F) ILC2 also expressed the pro-resolving receptors ALX (left panel) and CMKLR1 (right panel) on their surface (see Methods).
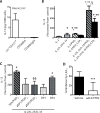
Fig. 5. IL-13 production by peripheral blood ILC2 is enhanced by PGD2and inhibited by lipoxin A4
IL-13 was quantitated by ELISA in the cell-free supernatants from incubations with (A) Lin−CD127+ cells, CD56bright NK cells and CD56dim NK cells that were sorted by flow cytometry and cultured for 24h alone (Control) or with PMA (50 ng/ml) and A23187 (500 ng/ml). Results are expressed per 1,000 cells. (B) In separate incubations, IL-13 release was determined for Lin−CD127+ cells that were incubated (15 min, 37°C) with lipoxin A4 (100 nM) or vehicle (0.1% ethanol) prior to (24h, 37°C) media alone, IL-2 (2 ng/ml) (IL-2), IL-2 (2 ng/ml), IL-25 (50 ng/ml) and IL-33 (50 ng/ml) (IL-2/IL-25/IL-33), IL-2 (2 ng/ml) and PGD2 (100 nM) (IL2/PGD2), or IL-2 (2 ng/ml), IL-25 (50 ng/ml), IL-33 (50 ng/ml) and PGD2 (100 nM) (IL-2/IL-25/IL-33/PGD2). (C) To assess PGD2 receptor signaling, ILCs were incubated with IL-2/IL-25/IL-33/PGD2 in the presence of a DP1 receptor antagonist (BWA868C, aDP1) or DP2 receptor antagonist (BAYu3405, aDP2). Some cells were exposed to IL-2/IL-25/IL-33 in conjunction with a selective DP1 receptor agonist (BW245C, DP1) or DP2 receptor agonist (15(R)-methyl-PGD2, DP2) without PGD2. (D) To determine the role of ALX/FPR2 signaling, cells were exposed (15 min, 37°C) to the receptor antagonist WRW4 (aALX/FPR2) prior to LXA4 and IL-2/IL-25/IL-33/PGD2. Results are expressed as the percent increase from control (mean ± s.e.m.); n≥3 individual healthy donors; *, P < 0.01, compared with control; **, P < 0.05, compared with IL-2; §, P < 0.05, compared with IL-2/IL-25/IL-33, §§, P < 0.05, compared with IL-2/IL-25/IL-33/PGD2; ***, P < 0.05, compared with vehicle.
Fig. 6. Innate lymphoid cells are present in human lung
Immune cells were assessed in the distal lung using immunofluorescent staining with antibodies for cKit, CD161, and perforin to identify cells with the staining characteristics of ILC (cKit+, CD161+) and NK cells (cKit−, CD161+, perforin+). Cell nuclei were stained with DAPI (blue). Panels on the left show cells that stain positively for ckit (green, Panel A) and CD161 (red, Panel B). When images A and B are merged, cells stain for ckit and CD161 (yellow, Panel C) or ckit alone (green, Panel C). Light microscopy images taken with 20X objective, and 100X objective for inset. Panels on the right show cells that that stain positively for ckit (green, Panel D) or Perforin (white, Panel D). Panel E depicts cells that are positive for Perforin (white), CD161 (red), or both Perforin and CD161 (white and red). When images D and E are merged, cells are present in Panel F that stains for Perforin and CD161 (white and red), Perforin alone (white), ckit alone (green), or ckit and CD161 (yellow). Confocal images taken with 60X objective. Inset images provide higher power views of the cells, and correspond numerically with the lower power images. Br, bronchioles
Comment on
- A new horizon in asthma: inhibiting ILC function.
Peebles RS Jr. Peebles RS Jr. Sci Transl Med. 2013 Feb 27;5(174):174fs7. doi: 10.1126/scitranslmed.3005881. Sci Transl Med. 2013. PMID: 23447016 Review.
Similar articles
- Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia.
Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O'Byrne PM, Gauvreau GM, Boulet LP, Lemiere C, Martin J, Nair P, Sehmi R. Smith SG, et al. J Allergy Clin Immunol. 2016 Jan;137(1):75-86.e8. doi: 10.1016/j.jaci.2015.05.037. Epub 2015 Jul 17. J Allergy Clin Immunol. 2016. PMID: 26194544 - Type 2 innate lymphoid cells-new members of the "type 2 franchise" that mediate allergic airway inflammation.
Mjösberg J, Spits H. Mjösberg J, et al. Eur J Immunol. 2012 May;42(5):1093-6. doi: 10.1002/eji.201242549. Eur J Immunol. 2012. PMID: 22539283 - MicroRNA-155 is a critical regulator of type 2 innate lymphoid cells and IL-33 signaling in experimental models of allergic airway inflammation.
Johansson K, Malmhäll C, Ramos-Ramírez P, Rådinger M. Johansson K, et al. J Allergy Clin Immunol. 2017 Mar;139(3):1007-1016.e9. doi: 10.1016/j.jaci.2016.06.035. Epub 2016 Aug 1. J Allergy Clin Immunol. 2017. PMID: 27492144 - Group 2 innate lymphoid cells (ILC2s): The spotlight in asthma pathogenesis and lung tissue injury.
Sadik S, Lu Y, Zhu S, Cai J, Mi LL. Sadik S, et al. Allergol Immunopathol (Madr). 2021 Mar 1;49(2):208-216. doi: 10.15586/aei.v49i2.29. eCollection 2021. Allergol Immunopathol (Madr). 2021. PMID: 33641310 Review. - Type 2 innate lymphoid cells: at the cross-roads in allergic asthma.
van Rijt L, von Richthofen H, van Ree R. van Rijt L, et al. Semin Immunopathol. 2016 Jul;38(4):483-96. doi: 10.1007/s00281-016-0556-2. Epub 2016 Mar 10. Semin Immunopathol. 2016. PMID: 26965110 Free PMC article. Review.
Cited by
- Emergence of Biomolecular Pathways to Define Novel Asthma Phenotypes. Type-2 Immunity and Beyond.
Wenzel SE. Wenzel SE. Am J Respir Cell Mol Biol. 2016 Jul;55(1):1-4. doi: 10.1165/rcmb.2016-0141PS. Am J Respir Cell Mol Biol. 2016. PMID: 27164162 Free PMC article. - Innate lymphocyte cells in asthma phenotypes.
Ozyigit LP, Morita H, Akdis M. Ozyigit LP, et al. Clin Transl Allergy. 2015 Jul 6;5:23. doi: 10.1186/s13601-015-0068-5. eCollection 2015. Clin Transl Allergy. 2015. PMID: 26150907 Free PMC article. Review. - TH2, allergy and group 2 innate lymphoid cells.
Licona-Limón P, Kim LK, Palm NW, Flavell RA. Licona-Limón P, et al. Nat Immunol. 2013 Jun;14(6):536-42. doi: 10.1038/ni.2617. Nat Immunol. 2013. PMID: 23685824 Review. - Pulmonary Administration of TLR2/6 Agonist after Allergic Sensitization Inhibits Airway Hyper-Responsiveness and Recruits Natural Killer Cells in Lung Parenchyma.
Devulder J, Barrier M, Carrard J, Amniai L, Plé C, Marquillies P, Ledroit V, Ryffel B, Tsicopoulos A, de Nadai P, Duez C. Devulder J, et al. Int J Mol Sci. 2024 Sep 4;25(17):9606. doi: 10.3390/ijms25179606. Int J Mol Sci. 2024. PMID: 39273551 Free PMC article. - Emerging concepts and future challenges in innate lymphoid cell biology.
Tait Wojno ED, Artis D. Tait Wojno ED, et al. J Exp Med. 2016 Oct 17;213(11):2229-2248. doi: 10.1084/jem.20160525. Epub 2016 Oct 10. J Exp Med. 2016. PMID: 27811053 Free PMC article. Review.
References
- Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. - PubMed
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. - PubMed
- Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, Castro M, Chung KF, Gaston B, Jarjour NN, Busse WW, Wenzel SE, Levy BD. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- P50 HL107166/HL/NHLBI NIH HHS/United States
- R01 AI068084/AI/NIAID NIH HHS/United States
- P01 GM095467/GM/NIGMS NIH HHS/United States
- R01 HL068669/HL/NHLBI NIH HHS/United States
- AI068084/AI/NIAID NIH HHS/United States
- HL109172/HL/NHLBI NIH HHS/United States
- HL102225/HL/NHLBI NIH HHS/United States
- HL107166/HL/NHLBI NIH HHS/United States
- U10 HL109172/HL/NHLBI NIH HHS/United States
- R56 AI068084/AI/NIAID NIH HHS/United States
- U01 HL102225/HL/NHLBI NIH HHS/United States
- R01 HL090927/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical