Glycyrrhizic acid as the antiviral component of Glycyrrhiza uralensis Fisch. against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease - PubMed (original) (raw)
Glycyrrhizic acid as the antiviral component of Glycyrrhiza uralensis Fisch. against coxsackievirus A16 and enterovirus 71 of hand foot and mouth disease
Jingjing Wang et al. J Ethnopharmacol. 2013.
Abstract
Ethnopharmacological relevance: The radices of Glycyrrhiza uralensis Fisch. and herbal preparations containing Glycyrrhiza spp. have been used for thousands of years as an herbal medicine for the treatment of viral induced cough, viral hepatitis, and viral skin diseases like ulcers in China. Glycyrrhizic acid (GA) is considered the principal component in Glycyrrhiza spp. with a wide spectrum of antiviral activity.
Aim: The present study attempt to validate the medicinal use of Glycyrrhiza uralensis for hand, foot and mouth disease (HFMD) and further to verify whether GA is an active antiviral component in the water extract of Glycyrrhiza uralensis.
Materials and methods: Radices of Glycyrrhiza uralensis Fisch. were extracted with hot water. The chemical contents of the extract were profiled with HPLC analysis. The antiviral activity of the extract and the major components was evaluated against infection of enterovirus 71 (EV71) and coxsackievirus A16 (CVA16) on Vero cells. The cytopathic effect caused by the infection was measured with MTT assay. Infectious virion production was determined using secondary infection assays and viral protein expression by immunoblotting analysis.
Results: The extract at 1000 μg/ml suppressed EV71 replication by 1.0 log and CVA16 by 1.5 logs. The antiviral activity was associated with the content of GA in the extract since selective depletion of GA from the extract by acid precipitation resulted in loss of antiviral activity. In contrast, the acid precipitant retained antiviral activity. The precipitant at a concentration of 200 μg/ml inhibited EV71 and CVA16 replication by 1.7 and 2.2 logs, respectively. Furthermore, GA dose-dependently blocked viral replication of EV71 and CVA16. At 3 mM, GA reduced infectious CVA16 and EV71 production by 3.5 and 2.2 logs, respectively. At 5mM, CVA16 production was reduced by 6.0 logs and EV71 by 4.0 logs. Both EV71 and CVA16 are members of Enterovirus genus, time-of-drug addition studies however showed that GA directly inactivated CVA16, while GA anti-EV71 effect was associated with an event(s) post virus cell entry.
Conclusions: This study validated the medicinal usefulness of radices Glycyrrhiza uralensis against the etiological agents of HFMD. In addition to the identification of GA as the antiviral component of Glycyrrhiza uralensis against EV71 and CVA16 infection, this study also reveals that GA inhibits EV71 and CVA16 with distinct mechanisms.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Figures
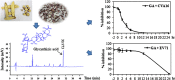
Graphical abstract
Fig. 1
HPLC profiling of the water extract of Glycyrrhiza uralensis. The radices of Glycyrrhiza uralensis were ground and extracted with diH2O. The extract was profiled on a BosChroma ODS-AQ column (4.6×150 mm, 5 mm particle size), using acetonitrile and H2O containing 0.1% trifluoroacetic acid (32:68) as a mobile phase. Liquiritin and glycyrrhizic acid were detected at 13.440 and 30.932 min with a UV detector. Upper panel is HPLC chromatogram of standard compounds; lower panel is a sample of Glycyrrhiza uralensis water extract.
Fig. 2
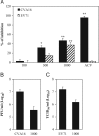
Evaluation of antiviral activity of a water extract of Glycyrrhiza uralensis against coxsackievirus A16 and enterovirus 71. (A) Antiviral activity of Glycyrrhiza uralensis water extract. Vero cells in triplicate samples were pretreated with the water extract of Glycyrrhiza uralensis at 100, 300, and 1000 μg/ml for 2 h or mock treated, the cells were then infected with CVA16 or EV71 (both at MOI=0.3) for 72 h. Cytopathic effect due to virus infection was determined with an MTT assay. The inhibition rates, presented as a percentage, were calculated as described in Section 2. Acyclovir (ACV) at 10 μM was used as an antiviral positive control for CVA16. Statistical significance was determined by one-way ANOVA (* denotes P<0.05 and ** denotes P<0.01). (B, C) Glycyrrhiza uralensis water extract treatment reduces infectious virion production. Vero cells were treated with the extract at 1000 μg/ml or mock treated for 2 h. The cells were then infected with CVA16 (B) or EV71 (C). The infected cells and the culture supernatants were harvested at 48 h PI. Infectious virions in those samples were titrated with secondary infection assays. Data are presented as mean±standard errors of triplicate samples. The results are representative of three independent experiments.
Fig. 3
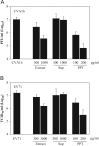
The water extract is devoid of antiviral activity after depletion of glycyrrhizic acid by acid precipitation; whereas the precipitant has improved antiviral activity. The pH of the water extract of Glycyrrhiza uralensis was lowered with dilute H2SO4 to precipitate glycyrrhizic acid using a standard protocol. The precipitant was collected by filtration. After neutralizing the pH of the supernatant devoid of glycyrrhizic acid, the antiviral activity against CVA16 (A) and EV71 (B) of this supernatant (Sup), the precipitant (PPT), and the water extract of Glycyrrhiza uralensis (Extract) was assayed on Vero cells. Acid precipitation removes glycyrrhizic acid from the water extract of Glycyrrhiza uralensis, resulting in loss of antiviral activity of the supernatant. As expected, the antiviral activity was enriched in the precipitant. Data are presented as mean±standard errors of triplicate samples from three independent experiments.
Fig. 4
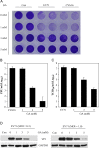
Inhibition of coxsackievirus A16 and enterovirus 71 infection by glycyrrhizic acid. (A) Glycyrrhizic acid treatment blocks the cytopathic effect by virus infection. Vero cells in duplicate were untreated or treated with glycyrrhizic acid at concentrations as indicated for 2 h. The cells were then infected with CVA16 or EV71 (MOI=0.3). The cells were fixed with 3% paraformaldehyde at 72 h PI and stained with 0.5% crystal violet. Inhibition of CVA16 or EV71 infection prevents cell death and hence increased staining compared infected but untreated controls. The results are representative from two independent experiments. (B, C) Glycyrrhizic acid treatment reduces infectious virion production. To assay for inhibition of virion production, Vero cells were treated with glycyrrhizic acid at 1, 3, and 5 mM or remained untreated. The cells were then infected with CVA16 (B) or with EV71 (C) at MOI=0.3. The samples were harvested at 48 h PI and infectious virions in those samples were assayed with secondary infection assays. Data are presented as mean±standard errors of triplicate samples from three independent experiments. (D) Glycyrrhizic acid treatment inhibits VP1 protein expression of EV71. Vero cells were infected with EV71 in the presence or absence of glycyrrhizic acid at concentrations as indicated. The cells were harvested at 48 h PI and VP1 expression in the samples was detected by immunoblotting analysis. GAPDH was used as a loading control. The results are representative from two independent experiments.
Fig. 5
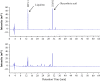
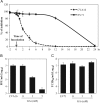
Glycyrrhizic acid inhibition of coxsackievirus A16 infection by direct inactivation, while the inhibition of enterovirus 71 infection is linked to a post cell entry event. (A) Time-of-drug addition study. Glycyrrhizic acid at 5 mM was added at 2 h prior to (−2 h), during (0 h), or post virus inoculation at time points as indicated (0.5 h, 1 h, 2 h, 4 h, 6 h, 12 h and 24 h PI). Virus infection was determined at 72 h PI using MTT assay. An inhibition rate was calculated as described in Section 2 and was plotted against time-of-drug addition. Data are presented as mean±standard errors of triplicate samples. The results are representative of two independent experiments. (B, C) Glycyrrhizic acid directly deactivates coxsackievirus A16, but not EV71. Duplicate samples of CVA16 (B) or EV71 (C) in 100 μl culture medium were incubated with glycyrrhizic acid at 0, 3, and 5 mM in a 37 °C water bath for 60 min. Ten microliters of the pre-treated virus or equal amount of untreated virus (final MOI=0.3) were then added to Vero cells (final concentrations of GA applied to Vero cells were 0.03 and 0.05 mM for GA-treated samples). Preincubation of CVA16 with 5 mM GA resulted in a loss of infectious virion production by more than 3 logs, whereas the same treatment did not significantly affect EV71 infectivity. Data are presented as mean±standard errors of duplicate samples. The results are representative of two independent experiments.
Similar articles
- Lycorine Derivative LY-55 Inhibits EV71 and CVA16 Replication Through Downregulating Autophagy.
Wang H, Guo T, Yang Y, Yu L, Pan X, Li Y. Wang H, et al. Front Cell Infect Microbiol. 2019 Aug 7;9:277. doi: 10.3389/fcimb.2019.00277. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31448243 Free PMC article. - Antiviral activity of Paulownia tomentosa against enterovirus 71 of hand, foot, and mouth disease.
Ji P, Chen C, Hu Y, Zhan Z, Pan W, Li R, Li E, Ge HM, Yang G. Ji P, et al. Biol Pharm Bull. 2015;38(1):1-6. doi: 10.1248/bpb.b14-00357. Biol Pharm Bull. 2015. PMID: 25744451 - Water extract of Glycyrrhiza uralensis inhibited enterovirus 71 in a human foreskin fibroblast cell line.
Kuo KK, Chang JS, Wang KC, Chiang LC. Kuo KK, et al. Am J Chin Med. 2009;37(2):383-94. doi: 10.1142/S0192415X09006904. Am J Chin Med. 2009. PMID: 19507280 - Activation of Host Cellular Signaling and Mechanism of Enterovirus 71 Viral Proteins Associated with Hand, Foot and Mouth Disease.
Swain SK, Panda S, Sahu BP, Sarangi R. Swain SK, et al. Viruses. 2022 Oct 4;14(10):2190. doi: 10.3390/v14102190. Viruses. 2022. PMID: 36298746 Free PMC article. Review. - A review on current diagnostic tools and potential optical absorption spectroscopy for HFMD detection.
Mustafa FH, Ismail I, Ahmad Munawar AAZ, Abdul Basir B, Shueb RH, Irekeola AA, Wan Ismail WZ, Jamaludin J, Balakrishnan SR, Sahrim M, Yusof NY. Mustafa FH, et al. Anal Biochem. 2023 Dec 15;683:115368. doi: 10.1016/j.ab.2023.115368. Epub 2023 Oct 25. Anal Biochem. 2023. PMID: 37890549 Review.
Cited by
- Effect of supplementation with Glycyrrhiza uralensis extract and Lactobacillus acidophilus on growth performance and intestinal health in broiler chickens.
Li X, Li J, Yuan H, Chen Y, Li S, Jiang S, Zha Xi Y, Zhang G, Lu J. Li X, et al. Front Vet Sci. 2024 Jul 18;11:1436807. doi: 10.3389/fvets.2024.1436807. eCollection 2024. Front Vet Sci. 2024. PMID: 39091388 Free PMC article. - The State-of-the-Art Antibacterial Activities of Glycyrrhizin: A Comprehensive Review.
Chen RY, Shi JJ, Liu YJ, Yu J, Li CY, Tao F, Cao JF, Yang GJ, Chen J. Chen RY, et al. Microorganisms. 2024 Jun 6;12(6):1155. doi: 10.3390/microorganisms12061155. Microorganisms. 2024. PMID: 38930536 Free PMC article. Review. - Exploring the pharmacological mechanism of Glycyrrhiza uralensis against KOA through integrating network pharmacology and experimental assessment.
Xu J, Sun Q, Qiu M, Wu Y, Cheng L, Jiang N, Zhang R, Chen J, Yuan W, Jin H, Wang W, Cai Y, Zhang C, Wang P. Xu J, et al. J Cell Mol Med. 2024 May;28(9):e18319. doi: 10.1111/jcmm.18319. J Cell Mol Med. 2024. PMID: 38742846 Free PMC article. - Licorice extract inhibits porcine epidemic diarrhea virus in vitro and in vivo.
Bai W, Zhu Q, Wang J, Jiang L, Guo D, Li C, Xing X, Sun D. Bai W, et al. J Gen Virol. 2024 Mar;105(3):001964. doi: 10.1099/jgv.0.001964. J Gen Virol. 2024. PMID: 38471043 Free PMC article. - Promising role of Vitamin D and plant metabolites against COVID-19: Clinical trials review.
Srivastava R, Singh N, Kanda T, Yadav S, Yadav S, Choudhary P, Atri N. Srivastava R, et al. Heliyon. 2023 Oct 21;9(11):e21205. doi: 10.1016/j.heliyon.2023.e21205. eCollection 2023 Nov. Heliyon. 2023. PMID: 37920525 Free PMC article. Review.
References
- Andersen F.A. Final report on the safety assessment of glycyrrhetinic acid, potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate, glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate, dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate. International Journal of Toxicology. 2007;26:79–112. - PubMed
- Cao H.J., Liu Z.L., Steinmann P., Mu Y.J., Luo H., Liu J.P. Chinese herbal medicines for treatment of hand, foot and mouth disease: a systematic review of randomized clinical trials. European Journal of Integrative Medicine. 2012;4:e85–e111.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous