Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro - PubMed (original) (raw)
doi: 10.1016/j.bbi.2013.02.005. Epub 2013 Feb 27.
Tifenn Le Charpentier, Sophie Lebon, Marie-Virgine Oré, Idoia Lara Celador, Julien Josserand, Vincent Degos, Etienne Jacotot, Henrik Hagberg, Karin Sävman, Carina Mallard, Pierre Gressens, Bobbi Fleiss
Affiliations
- PMID: 23454862
- PMCID: PMC3694309
- DOI: 10.1016/j.bbi.2013.02.005
Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro
Vibol Chhor et al. Brain Behav Immun. 2013 Aug.
Abstract
Microglia mediate multiple facets of neuroinflammation, including cytotoxicity, repair, regeneration, and immunosuppression due to their ability to acquire diverse activation states, or phenotypes. Modulation of microglial phenotype is an appealing neurotherapeutic strategy but a comprehensive study of classical and more novel microglial phenotypic markers in vitro is lacking. The aim of this study was to outline the temporal expression of a battery of phenotype markers from polarised microglia to generate an in vitro tool for screening the immunomodulatory potential of novel compounds. We characterised expression of thirty-one macrophage/microglial phenotype markers in primary microglia over time (4, 12, 36, and 72 h), using RT-qPCR or multiplex protein assay. Firstly, we selected Interleukin-4 (IL-4) and lipopolysaccharide (LPS) as the strongest M1-M2 polarising stimuli, from six stimuli tested. At each time point, markers useful to identify that microglia were M1 included iNOS, Cox-2 and IL-6 and a loss of M2a markers. Markers useful for quantifying M2b-immunomodulatory microglia included, increased IL-1RA and SOCS3 and for M2a-repair and regeneration, included increased arginase-1, and a loss of the M1 and M2b markers were discriminatory. Additional markers were regulated at fewer time points, but are still likely important to monitor when assessing the immunomodulatory potential of novel therapies. Further, to facilitate identification of how novel immunomodulatory treatments alter the functional affects of microglia, we characterised how the soluble products from polarised microglia affected the type and rate of neuronal death; M1/2b induced increasing and M2a-induced decreasing neuronal loss. We also assessed any effects of prior activation state, to provide a way to identify how a novel compound may alter phenotype depending on the stage of injury/insult progression. We identified generally that a prior M1/2b reduced the ability of microglia to switch to M2a. Altogether, we have characterised a profile of phenotype markers and a mechanism of assessing functional outcome that we can use as a reference guide for first-line screening of novel immunomodulatory therapies in vitro in the search for viable neuroprotectants.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Supplementary Fig. 1
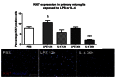
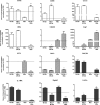
In response to LPS gene expression for M1 and M2b are induced within 2 h of stimulation. Gene expression of markers categorized as M1 (white bars), M2a (grey bars) and M2b (black bars) in primary microglia exposed to M1/2b inducing condition (+LPS). Expression shown relative to PBS only, dotted line. Data are mean ± SEM of at least 3 independent experiments. Data were assessed via an ANOVA, and where significant the results of a Bonferroni post-test are shown; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001, compared to PBS.
Supplementary Fig. 2
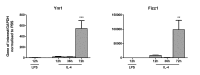
Expression of YM1 and Fizz1 are increased after 72 h of M2a inducing conditions. Gene expression for YM1 and Fizz1 in primary microglia stimulated for 12, 36, or 72 hr with either LPS or IL4. Data are mean ± SEM of at least 3 independent experiments assessed via a Kruskal Wallis, and where significant the results of a Dunn’s post test are indicated; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 compared to averaged PBS in post-hoc.
Supplementary Fig. 3
Expression of CD86 did not vary dramatically, and Arg1 increased in microglia exposed to IL4. Microglia were stimulated for 36 h with LPS, IL-4 or with PBS control. CD86 staining in green and Arg1 in red. Photomicrographs taken at 63×, scale bar 25 μm.
Supplementary Fig. 4
Proliferation was altered when microglial phenotype was polarized, LPS inducing and IL-4 reducing Ki67 expression. Microglia were stained with Ki67 (red) and Hoechst (blue) after exposure for 12 or 36 h to LPS or IL-4, with PBS as control. Ki67 positive cell numbers were counted and expressed as a proportion of total cell number (Hoechst positive). Photomicrograph were taken at 60× magnification, scale bar 100 μm.
Supplementary Fig. 5
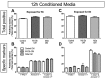
Cell death was largely unaffected by exposure to soluble factors from M1/2a or M2b microglia for 5 or 8 h. Total appearance of cell death markers (top panels) and relative numbers of specific cell death type markers (middle panels) in neurons exposed to conditioned media harvested following 12 h induction to an M1/2b or M2a phenotype, or exposure to PBS. Neuronal viability was assessed after exposure of neurons for 5 h (A, B) or exposure for 8 h (C, D) to the conditioned media. Viability was assessed dependent on positive staining for 7-ADD (necrotic-like death marker), Annexin V (apoptotic-like death marker), or both (late stage cell death indicated).
Fig. 1
Schematic representation of the experimental setups, including phenotyping, neuronal viability and phenotyping switching experiments.
Fig. 2
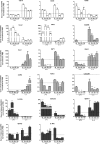
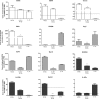
Gene expression of phenotype markers over time in response to M1 or M2 inducing conditions. Expression of phenotype markers grouped as M1 (White), M2a (grey) or M2b (black) dependent on their proposed function, in primary microglia exposed to M1/2b inducing conditions (+LPS) or M2a inducing conditions (+IL-4), for 4 h, 12 h, 36 h or 72 h. Expression shown relative to PBS only, dotted line. Data are mean ± SEM of at least 3 experiments. Data were assessed via an ANOVA, and where significant the results of the Bonferroni post-test are shown; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001, compared to PBS.
Fig. 3
Protein expression for phenotype markers over time in response to M1 or M2 inducing conditions. Expression of phenotype markers in the conditioned media, grouped as M1 (White), M2a (grey) or M2b (black) dependent on their proposed function, from primary microglia exposed to M1/2b inducing conditions (+LPS) or M2b inducing conditions (+IL-4), for 4 h, 12 h, 36 h or 72 h. Expression shown relative to PBS only, dotted line. Data are mean ± SEM of at least 3 experiments. Data were assessed via an ANOVA, and where significant the results of a Bonferroni post-test are shown; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001, compared to PBS.
Fig. 4
Expression of selected phenotype markers in cultured microglia. Microglia were stained with markers as indicated after exposure for 12 or 36 h to LPS or IL-4, with PBS as control. All photomicrograph were taken at 63× magnification, scale bar 25 μm. Panels A–F, Cox-2 in red, IL-1RA in green and Hoechst in blue. Panels G–L, Arg1 in red, iNOS in green and DAPI in blue.
Fig. 5
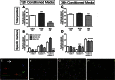
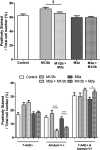
Phenotype of microglia alters the neuronotoxicity of conditioned media. Total appearance of cell death markers (A, C) and relative numbers of specific cell death type markers (B, D) in neurons exposed for 14 h to conditioned media harvested following 12 h (A, B, F–H) or 36 h (C, D) induction to an M1/2b or M2a phenotype, or PBS. Viability was assessed dependent on positive staining for 7-ADD, Annexin V, or both (see example, E), and specifically neurons were categorised as ‘normal’ (blue arrow) double negative and Hoechst positive, Annexin V positive only (red arrow), 7AAD+ positive only (green arrow), Annexin V+ and 7AAD+ with uncondensed nuclei (white arrow) or Annexin V+ and 7AAD+ condensed nuclei (orange arrow). Cells double positive with an uncondensed nuclei (white arrow) were very extremely rare. Representative images taken after exposure of neurons to 12 h CM for 14 h (F–H), illustrating basal staining of neurons in F (+PBS CM), increased cell death in +LPS CM (G), and deceased cell death in +IL-4 CM (H). Scale bar 25 μm. Data are mean ± SEM of at least 3 experiments. Data were assessed via an Kruskal Wallis, and where significant results of a Dunn’s post-test are shown; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001, compared to PBS.
Fig. 6
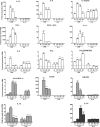
Prior phenotype alters the expression of phenotype markers in response to a second stimuli. Microglia were exposed to a single stimulus (LPS for 12 h or IL-4 for 36 h) or after one stimulus was removed it was replaced with the alternate stimulus as indicated. Gene expression is shown for markers grouped as M1 (white), M2a (grey) or M2b (black) dependent on their proposed function. Expression shown relative to PBS only treated control values from the respective time points, dotted line. Data are mean ± SEM of at least 3 independent experiments and assessed via Student’s t_-test (primary stimuli vs. switched); ∗_p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Fig. 7
Prior IL-10 exposure impairs the development of a later M1/2b phenotype. Microglia were exposed to single or consecutive treatments of LPS (12 h) or IL-10 (12 h) as indicated and shown is gene expression for markers grouped as M1 (White), M2a (grey) or M2b (black) dependent on their proposed function. Expression shown relative to PBS only treated control values from the respective time points, dotted line. Data are mean ± SEM of at least 3 experiments assessed via Student’s t-test (primary stimuli vs. switched); ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Fig. 8
Prior phenotype reduces the neuronotoxicity of soluble factors from cytotoxic microglia. Neurons were exposed to conditioned media from microglia induced to a phenotype with a single stimulus (LPS 12 h or IL-4 36 h), or where the two treatments were applied in succession, with media changed between stimuli. After exposure to conditioned media for 14 h, the number of cells positive for cell death markers 7-AAD or Annexin V was assessed relative to total cell number (Hoechst positive). Data are mean ± SEM of at least 3 independent experiments assessed via Mann Whitney (single stimulus vs. switched); §p = 0.05; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Similar articles
- Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines.
Ghosh M, Xu Y, Pearse DD. Ghosh M, et al. J Neuroinflammation. 2016 Jan 13;13:9. doi: 10.1186/s12974-015-0463-9. J Neuroinflammation. 2016. PMID: 26757726 Free PMC article. - Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells.
Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J. Zhang J, et al. Mol Immunol. 2019 Dec;116:29-37. doi: 10.1016/j.molimm.2019.09.020. Epub 2019 Oct 4. Mol Immunol. 2019. PMID: 31590042 - Anthocyanin effects on microglia M1/M2 phenotype: Consequence on neuronal fractalkine expression.
Meireles M, Marques C, Norberto S, Santos P, Fernandes I, Mateus N, Faria A, Calhau C. Meireles M, et al. Behav Brain Res. 2016 May 15;305:223-8. doi: 10.1016/j.bbr.2016.03.010. Epub 2016 Mar 7. Behav Brain Res. 2016. PMID: 26965567 - Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains.
Walker DG, Lue LF. Walker DG, et al. Alzheimers Res Ther. 2015 Aug 19;7(1):56. doi: 10.1186/s13195-015-0139-9. Alzheimers Res Ther. 2015. PMID: 26286145 Free PMC article. Review. - Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases.
Tang Y, Le W. Tang Y, et al. Mol Neurobiol. 2016 Mar;53(2):1181-1194. doi: 10.1007/s12035-014-9070-5. Epub 2015 Jan 20. Mol Neurobiol. 2016. PMID: 25598354 Review.
Cited by
- Neuroinflammation in early, late and recovery stages in a progressive parkinsonism model in rats.
Cunha DMG, Becegato M, Meurer YSR, Lima AC, Gonçalves N, Bioni VS, Engi SA, Bianchi PC, Cruz FC, Santos JR, Silva RH. Cunha DMG, et al. Front Neurosci. 2022 Aug 26;16:923957. doi: 10.3389/fnins.2022.923957. eCollection 2022. Front Neurosci. 2022. PMID: 36090265 Free PMC article. - Differential cytokine expression by brain microglia/macrophages in primary culture after oxygen glucose deprivation and their protective effects on astrocytes during anoxia.
Barakat R, Redzic Z. Barakat R, et al. Fluids Barriers CNS. 2015 Feb 28;12:6. doi: 10.1186/s12987-015-0002-1. eCollection 2015. Fluids Barriers CNS. 2015. PMID: 25866619 Free PMC article. - The interplay between cytokines and stroke: a bi-directional Mendelian randomization study.
Jiang Y, Liu Q, Wang C, Zhao Y, Jin C, Sun M, Ge S. Jiang Y, et al. Sci Rep. 2024 Jul 26;14(1):17657. doi: 10.1038/s41598-024-67615-4. Sci Rep. 2024. PMID: 39085243 Free PMC article. - [Exosomes from ectoderm mesenchymal stem cells inhibits lipopolysaccharide-induced microglial M1 polarization and promotes survival of H2O2-exposed PC12 cells by suppressing inflammatory response and oxidative stress].
Sun X, Shi H, Zhang L, Liu Z, Li K, Qian L, Zhu X, Yang K, Fu Q, Ding H. Sun X, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2024 Jan 20;44(1):119-128. doi: 10.12122/j.issn.1673-4254.2024.01.14. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 38293983 Free PMC article. Chinese. - The Interplay between Ghrelin and Microglia in Neuroinflammation: Implications for Obesity and Neurodegenerative Diseases.
Russo C, Valle MS, Russo A, Malaguarnera L. Russo C, et al. Int J Mol Sci. 2022 Nov 3;23(21):13432. doi: 10.3390/ijms232113432. Int J Mol Sci. 2022. PMID: 36362220 Free PMC article. Review.
References
- Akuzawa S., Kazui T., Shi E., Yamashita K., Bashar A.H., Terada H. Interleukin-1 receptor antagonist attenuates the severity of spinal cord ischemic injury in rabbits. J. Vasc. Surg. 2008;48:694–700. - PubMed
- Aly H., Khashaba M.T., El-Ayouty M., El-Sayed O., Hasanein B.M. IL-1beta, IL-6 and TNF-alpha and outcomes of neonatal hypoxic ischemic encephalopathy. Brain Dev. 2006;28:178–182. - PubMed
- Bernardino L., Xapelli S., Silva A.P., Jakobsen B., Poulsen F.R., Oliveira C.R., Vezzani A., Malva J.O., Zimmer J. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J. Neurosci. 2005;25:6734–6744. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials