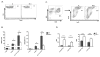Host b7x promotes pulmonary metastasis of breast cancer - PubMed (original) (raw)
Host b7x promotes pulmonary metastasis of breast cancer
Yael M Abadi et al. J Immunol. 2013.
Abstract
B7x (B7-H4 or B7S1) is an inhibitory member of the B7 family of T cell costimulation. It is expressed in low levels in healthy peripheral tissues, such as the lung epithelium, but is overexpressed in a variety of human cancers with negative clinical associations, including metastasis. However, the function of B7x in the context of cancer, whether expressed on cancer cells or on surrounding "host" tissues, has not been elucidated in vivo. We used the 4T1 metastatic breast cancer model and B7x knockout (B7x (-/-)) mice to investigate the effect of host tissue-expressed B7x on cancer. We found that 4T1 cells were B7x negative in vitro and in vivo, and B7x(-/-) mice had significantly fewer lung 4T1 tumor nodules than did wild-type mice. Furthermore, B7x(-/-) mice showed significantly enhanced survival and a memory response to tumor rechallenge. Mechanistic studies revealed that the presence of B7x correlated with reduced general and tumor-specific T cell cytokine responses, as well as with an increased infiltration of immunosuppressive cells, including tumor-associated neutrophils, macrophages, and regulatory T cells, into tumor-bearing lungs. Importantly, tumor-associated neutrophils strongly bound B7x protein and inhibited the proliferation of both CD4 and CD8 T cells. These results suggest that host B7x may enable metastasizing cancer cells to escape local antitumor immune responses through interactions with the innate and adaptive immune systems. Thus, targeting the B7x pathway holds much promise for improving the efficacy of immunotherapy for metastatic cancer.
Figures
Figure 1
4T1 cells are B7x negative and B7x knockout mice have reduced lung metastasis of 4T1. (A) 4T1 cells were stained with anti-B7x (open line) or isotype control (shaded line). (B) 4T1 cells were stimulated with IFN-γ (100 ng/ml) for three days and then stained with anti-B7x (open line), anti-PD-L1 (dash line) or isotype control (shaded line). (C) RT-PCR of mRNA isolated from naïve or 4T1 metastatic lung. (D) Thy1.1 positive cells (4T1) from tail-vein Thy1.1/4T1 injected lungs of wt and B7x−/− mice were stained with anti-B7x (open line) or isotype control (shaded line). (E, F) Lung metastases resulting from tail-vein 4T1 injection; representative lungs after India ink injection (E) and lung tumor nodule counts (F) pooled from 2 independent experiments, n=10–12. ***P<0.001.
Figure 2
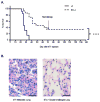
B7x knockout mice can survive 4T1 and 4T1 re-challenge. (A) Survival of mice injected with 4T1 cells in the tail-vein, n=13–15, representative of 2 separate experiments. At 71 d post-injection, remaining mice were re-challenged with double the number of 4T1 cells. (B) H&E staining of lung sections of the double-challenged mice at day 140 and other 4T1-injected mice. Left image: lung tissue bearing 4T1 metastasis. Right image: representative lung tissue from the double-challenged B7x−/− mice. ****P<0.0001. Scale bar = 25 μm, 40x.
Figure 3
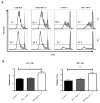
Analysis of lung immune cell infiltrate reveals higher cytokine responses to general and tumor-specific stimulation by T cells from knockout mice. (A) Numbers of immune effector cells infiltrating lungs at 17–18 d after 4T1 injection, pooled from 2–5 experiments, n=8–22. (B, C) PMA/ionomycin-stimulated T cells from lungs of 4T1-injected mice were analyzed for cytokine production. Percentages of IFN-γ+ or TNF-α+ cells are shown in representative FACS plots (B) and pooled from 3 independent experiments, each dot representing an individual mouse, n=11–12 (C). (D, E) CD8 T cell cytokine response after stimulation with 4T1 lysate-loaded DCs shown as representative FACS plots (D) and percentages of IFN-γ+ cells (E), n=4. *P<0.05; **P<0.01.
Figure 4
B7x knockout mice have fewer suppressive cells and a higher ratio of effector to suppressor cells. Cell suspensions from lungs and spleens of day 17–18 4T1-injected wt and B7x−/− mice were stained with lineage markers. (A) Representative FACS plots of permeablized CD4 cells stained for Foxp3. (B) Cell type numbers determined by flow cytometry, pooled from 4–5 independent experiments, n=16–22 (lungs, left) and 2–4 experiments, n=8–10 (spleens, right). (C) Representative FACS plots of CD11b+Ly6G+ cells among total CD45+ cells in wt and B7x−/− lungs. (D) The ratios of Foxp3-CD4+ (CD4eff) and CD8 T cells to CD11b+Ly6G+ (TAN) cells (left) and CD4eff and CD8 T cells to Foxp3+ CD4+ (Treg) cells (right) were calculated within each lung sample. Results are pooled from 4 independent experiments, n=16–18. *P<0.05; **P<0.01; ***P<0.001.
Figure 5
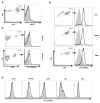
CD11b+Ly6G+ cells infiltrating metastatic wt and B7x−/− lungs are GR1hi morphologically mature neutrophils. (A) Metastatic lung cell suspensions were stained with lineage markers. Shown is a representative wt lung sample gated on CD45 and analyzed for CD11b, GR1, Ly6G, and Ly6C expression. (B) Ly6Ghi cells sorted from lungs of 4T1-injected wt and B7x−/− mice were stained with hematoxylin following Cytospin centrifugation. Representative images are shown, 40x.
Figure 6
Neutrophils isolated from tumor-bearing lungs suppress T cell proliferation. 105 CFSE-labeled T cells from naïve spleen were stimulated with plate-bound anti-CD3 alone or in the presence of 105 neutrophils purified from naïve bone marrow or from 4T1-metastatic wt or B7x−/− lungs. After 4 d, cells were stained with CD4, CD8, and Live/dead marker, and acquired by flow cytometry. (A) Representative FACS plots show CFSE dilution among live CD4-gated and CD8-gated stimulated (open line) compared to unstimulated control (shaded line) T cells. (B) Proliferative index of CD4 and CD8 T cells co-cultured with control or wt tumor associated neutrophils was calculated by FlowJo. Results are pooled from 2–3 independent experiments, n=3–10. *P<0.05; **P<0.01.
Figure 7
Tumor-associated neutrophils strongly bind B7x. (A) Single cell suspensions from lungs of wt 4T1-injected mice were stained with CD45 and anti-B7x (open line) or isotype control (shaded line). (B, C) Ly6G+ cells isolated from lung and spleen and RBC-depleted blood cells from 4T1-injected wt mice (B) and naïve wt and B7x−/− lung cell suspensions (C) were incubated with B7x-Ig (open line) or control Hu-IgG (shaded line) followed by staining with Ly6G and antibody recognizing the Ig portion of both fusion proteins (anti-HuIgG). (D) Cells were incubated with B7x-Ig (open line) or Hu-IgG (shaded line) and stained with anti-HuIgG. Histograms in A-C are representative of 2–5 separate experiments. Staining of HL-60 was repeated three times with similar results.
Similar articles
- Tissue-expressed B7x affects the immune response to and outcome of lethal pulmonary infection.
Hofmeyer KA, Scandiuzzi L, Ghosh K, Pirofski LA, Zang X. Hofmeyer KA, et al. J Immunol. 2012 Sep 15;189(6):3054-63. doi: 10.4049/jimmunol.1200701. Epub 2012 Aug 1. J Immunol. 2012. PMID: 22855708 Free PMC article. - Structure and cancer immunotherapy of the B7 family member B7x.
Jeon H, Vigdorovich V, Garrett-Thomson SC, Janakiram M, Ramagopal UA, Abadi YM, Lee JS, Scandiuzzi L, Ohaegbulam KC, Chinai JM, Zhao R, Yao Y, Mao Y, Sparano JA, Almo SC, Zang X. Jeon H, et al. Cell Rep. 2014 Nov 6;9(3):1089-98. doi: 10.1016/j.celrep.2014.09.053. Epub 2014 Oct 30. Cell Rep. 2014. PMID: 25437562 Free PMC article. - B7x/B7-H4 modulates the adaptive immune response and ameliorates renal injury in antibody-mediated nephritis.
Pawar RD, Goilav B, Xia Y, Herlitz L, Doerner J, Chalmers S, Ghosh K, Zang X, Putterman C. Pawar RD, et al. Clin Exp Immunol. 2015 Feb;179(2):329-43. doi: 10.1111/cei.12452. Clin Exp Immunol. 2015. PMID: 25205493 Free PMC article. - The B7x Immune Checkpoint Pathway: From Discovery to Clinical Trial.
John P, Wei Y, Liu W, Du M, Guan F, Zang X. John P, et al. Trends Pharmacol Sci. 2019 Nov;40(11):883-896. doi: 10.1016/j.tips.2019.09.008. Epub 2019 Oct 31. Trends Pharmacol Sci. 2019. PMID: 31677920 Free PMC article. Review. - Potential targeting of B7-H4 for the treatment of cancer.
Podojil JR, Miller SD. Podojil JR, et al. Immunol Rev. 2017 Mar;276(1):40-51. doi: 10.1111/imr.12530. Immunol Rev. 2017. PMID: 28258701 Free PMC article. Review.
Cited by
- Coinhibitory Pathways in the B7-CD28 Ligand-Receptor Family.
Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Schildberg FA, et al. Immunity. 2016 May 17;44(5):955-72. doi: 10.1016/j.immuni.2016.05.002. Immunity. 2016. PMID: 27192563 Free PMC article. Review. - B7 Family Members in Pancreatic Ductal Adenocarcinoma: Attractive Targets for Cancer Immunotherapy.
Chen X, Li J, Chen Y, Que Z, Du J, Zhang J. Chen X, et al. Int J Mol Sci. 2022 Nov 30;23(23):15005. doi: 10.3390/ijms232315005. Int J Mol Sci. 2022. PMID: 36499340 Free PMC article. Review. - Comparison of immunity in mice cured of primary/metastatic growth of EMT6 or 4THM breast cancer by chemotherapy or immunotherapy.
Gorczynski RM, Chen Z, Erin N, Khatri I, Podnos A. Gorczynski RM, et al. PLoS One. 2014 Nov 19;9(11):e113597. doi: 10.1371/journal.pone.0113597. eCollection 2014. PLoS One. 2014. PMID: 25409195 Free PMC article. - A SOX9-B7x axis safeguards dedifferentiated tumor cells from immune surveillance to drive breast cancer progression.
Liu Y, John P, Nishitani K, Cui J, Nishimura CD, Christin JR, Couturier N, Ren X, Wei Y, Pulanco MC, Galbo PM Jr, Zhang X, Fu W, Cui W, Bartholdy BA, Zheng D, Lauvau G, Fineberg SA, Oktay MH, Zang X, Guo W. Liu Y, et al. Dev Cell. 2023 Dec 4;58(23):2700-2717.e12. doi: 10.1016/j.devcel.2023.10.010. Epub 2023 Nov 13. Dev Cell. 2023. PMID: 37963469 Free PMC article. - Identification of the cis‑molecular neighbours of the immune checkpoint protein B7‑H4 in the breast cancer cell‑line SK‑BR‑3 by proteomic proximity labelling.
Rees JS, Cheung LCC, Hamaia SW, Davies G, Sandercock A, Lilley KS, Tigue N, Jackson AP. Rees JS, et al. Int J Oncol. 2020 Jul;57(1):87-99. doi: 10.3892/ijo.2020.5037. Epub 2020 Apr 3. Int J Oncol. 2020. PMID: 32319587 Free PMC article.
References
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
- de Souza AP, Bonorino C. Tumor immunosuppressive environment: effects on tumor-specific and nontumor antigen immune responses. Expert Rev Anticancer Ther. 2009;9:1317–1332. - PubMed
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM007288/GM/NIGMS NIH HHS/United States
- T32 DK007513/DK/NIDDK NIH HHS/United States
- T32GM007491/GM/NIGMS NIH HHS/United States
- T32 GM007491/GM/NIGMS NIH HHS/United States
- T32DK007218/DK/NIDDK NIH HHS/United States
- DP2 DK083076/DK/NIDDK NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- PC094137/PC/NCI NIH HHS/United States
- P30CA013330/CA/NCI NIH HHS/United States
- T32DK007513/DK/NIDDK NIH HHS/United States
- DP2DK083076/DK/NIDDK NIH HHS/United States
- T32 DK007218/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials