Exome sequencing identifies DYNC2H1 mutations as a common cause of asphyxiating thoracic dystrophy (Jeune syndrome) without major polydactyly, renal or retinal involvement - PubMed (original) (raw)
doi: 10.1136/jmedgenet-2012-101284. Epub 2013 Mar 1.
Heleen H Arts, Ernie M H F Bongers, Zhimin Yap, Machteld M Oud, Dinu Antony, Lonneke Duijkers, Richard D Emes, Jim Stalker, Jan-Bart L Yntema, Vincent Plagnol, Alexander Hoischen, Christian Gilissen, Elisabeth Forsythe, Ekkehart Lausch, Joris A Veltman, Nel Roeleveld, Andrea Superti-Furga, Anna Kutkowska-Kazmierczak, Erik-Jan Kamsteeg, Nursel Elçioğlu, Merel C van Maarle, Luitgard M Graul-Neumann, Koenraad Devriendt, Sarah F Smithson, Diana Wellesley, Nienke E Verbeek, Raoul C M Hennekam, Hulya Kayserili, Peter J Scambler, Philip L Beales; UK10K; Nine Vam Knoers, Ronald Roepman, Hannah M Mitchison
Collaborators, Affiliations
- PMID: 23456818
- PMCID: PMC3627132
- DOI: 10.1136/jmedgenet-2012-101284
Free PMC article
Exome sequencing identifies DYNC2H1 mutations as a common cause of asphyxiating thoracic dystrophy (Jeune syndrome) without major polydactyly, renal or retinal involvement
Miriam Schmidts et al. J Med Genet. 2013 May.
Free PMC article
Abstract
Background: Jeune asphyxiating thoracic dystrophy (JATD) is a rare, often lethal, recessively inherited chondrodysplasia characterised by shortened ribs and long bones, sometimes accompanied by polydactyly, and renal, liver and retinal disease. Mutations in intraflagellar transport (IFT) genes cause JATD, including the IFT dynein-2 motor subunit gene DYNC2H1. Genetic heterogeneity and the large DYNC2H1 gene size have hindered JATD genetic diagnosis.
Aims and methods: To determine the contribution to JATD we screened DYNC2H1 in 71 JATD patients JATD patients combining SNP mapping, Sanger sequencing and exome sequencing.
Results and conclusions: We detected 34 DYNC2H1 mutations in 29/71 (41%) patients from 19/57 families (33%), showing it as a major cause of JATD especially in Northern European patients. This included 13 early protein termination mutations (nonsense/frameshift, deletion, splice site) but no patients carried these in combination, suggesting the human phenotype is at least partly hypomorphic. In addition, 21 missense mutations were distributed across DYNC2H1 and these showed some clustering to functional domains, especially the ATP motor domain. DYNC2H1 patients largely lacked significant extra-skeletal involvement, demonstrating an important genotype-phenotype correlation in JATD. Significant variability exists in the course and severity of the thoracic phenotype, both between affected siblings with identical DYNC2H1 alleles and among individuals with different alleles, which suggests the DYNC2H1 phenotype might be subject to modifier alleles, non-genetic or epigenetic factors. Assessment of fibroblasts from patients showed accumulation of anterograde IFT proteins in the ciliary tips, confirming defects similar to patients with other retrograde IFT machinery mutations, which may be of undervalued potential for diagnostic purposes.
Figures
Figure 1
Mutations causing Jeune asphyxiating thoracic dystrophy (JATD). (A) Linear structure of the 4314 residue human DYNC2H1 protein showing the location of the 34 mutations described in this study in black, below the protein. Brackets indicate synonymous change associated with a splice site mutation. All previously reported DYNC2H1 mutations are shown above the protein, associated with JATD (black), short-rib polydactyly syndrome (SRPS II) (red) and SRPS III (orange). Conserved protein domains were taken from the consensus CDS entry for NP_001073932.1. The DYNC2H1 protein domains contain the six AAA+ domains of the hexomeric ring-like ATP-hydrolysing motor domain, AAA1-AAA6: AAA1 (amino acids 1651–1875), AAA2 (aa. 1938–2161), AAA3 (aa. 2251–2505), AAA4 (aa. 2617–2863), AAA5 (aa. 3244–3479) and AAA6 (aa. 3697–3912). In addition, other domain-associated structures allow DYNC2H1 to function as a motor: a thin microtubule binding stalk domain between AAA4 and AAA5 for attachment to microtubules (MT-binding stalk, aa. 2881–3227); an N-terminal tail (DHC_N1, aa. 234–676); and a linker domain (DHC_N2, aa. 1120–1520) thought to change position in different nucleotide states to create the powerstroke for motility along microtubules; plus a conserved C-terminal domain arranged on top of the ATPase ring (Dynein_heavy, aa. 3621–4311). (B) The predicted human DYNC2H1 protein is shown modelled to the resolved crystal structure of the cytoplasmic dynein heavy chain of Dictyostelium discoideum (DYHC_DICDI; PBD 3VKH) using Swiss-Model. Amino acid residues 1204–2969 could be modelled with confidence; AAA1, blue; AAA2, lime green; AAA3, red; AAA4, dark grey. The chain B of 3VKH used for the modelling is highlighted in light grey. (C) The location of the DYNC2H1 missense mutations that map to the regions of the protein that were possible to model by homology are shown in black. (D) The p.R2481Q substitution missense mutation could create a new hydrogen bond between Q2481 and the conserved tyrosine (TYR) at position Y2477.
Figure 2
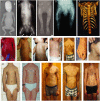
Clinical features of DYNC2H1 patients. (A–E) Hallmarks of Jeune asphyxiating thoracic dystrophy (JATD): (A, JATD-5; B, JATD-16) Small thorax due to short ribs; (A, JATD-5, B, JATD-16, C, JATD-5, D, JATD-14) Small ilia with acetabular spurs; (C, JATD-5, D, JATD-14) Shortening of femurs, accompanied by bowing in (D, JATD-14); (E) 3D reconstruction of CT images of patient JATD-4. (F–I) Severity of the rib shortening varies between different patients from different families carrying DYNC2H1 mutations as well as between affected siblings: while patient JATD-5 presents with extremely shortened ribs (F), patient JATD-18 (UCL62.2) is only mildly affected (G). (H, I) Patient JATD-14 (H, UCL80.1) is markably more severely affected than his sister JATD-14 (I, UCL80.2). (J–L) Additional features: (J) scoliosis in JATD-2, (K) syndactyly in JATD-2, (L) ear malformation in JATD-16. (M–Q) Thoracic narrowing becomes less pronounced with increasing patient age. (M) Shows patient JATD-16 at under 5 years; the same patient is shown a few years later in (N) at under 10 years. (O) Patient JATD-3 in his 20s, (P) patient JATD-2 in his late teens, (Q) patient JATD-1 in his mid-20s these cases have less pronounced thoracic phenotypes compared to birth or infancy, as described in the text. Note also that shortening of the upper limbs seems less severe when JATD patients reach adolescence.
Figure 3
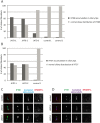
Intraflagellar transport (IFT-B) accumulations in ciliary tips in Jeune asphyxiating thoracic dystrophy (JATD) patient fibroblasts. (A) In contrast to wild type fibroblasts (controls 1 and 2) in which IFT88 localises primarily to the ciliary base (and to a much lesser amount to the tip), IFT88 concentrates distally in cilia in fibroblasts derived from JATD-1, -2 and -3 family patients. Cells that were analysed in JATD-3 are derived from affected individual II-4 (Table 1). Per condition at least 100 cells stained for IFT88, acetylated-α tubulin (marker for the ciliary axoneme) and RPGRIP1L (marker for the ciliary base) as displayed in panel C were independently analysed by two blinded researchers. The control fibroblast lines were derived from individuals unrelated to our JATD patients. The graph shows that 64%, 59% and 14% of cells from patients from families 1, -2 and -3 demonstrate IFT88 concentrations in ciliary tips, whereas this effect is only observed in 3% and 2% of the cells from controls 1 and 2, respectively. (B) Another IFT-B complex partner IFT57 also accumulates in ciliary tips of JATD fibroblasts. While 53% and 50% of ciliated fibroblasts from JATD-1 and -2 patients demonstrate IFT57 accumulations in the ciliary tips, only 2% of the cells of the control display this cellular phenotype (also see panel D). Cells were analysed as per (A). (C) Compared with controls, IFT88 accumulates in distal ends of cilia in fibroblasts from JATD-1 and -2 patients. The images show a single cilium per patient or control in detail. Cells were stained with anti-IFT88 (green); antiacetylated α tubulin (marker for the ciliary axoneme, cyan); and anti-RPGRIP1L (marker for the ciliary base, red). Whole-field images displaying multiple cilia are available in online
supplementary figure
S7. (D) Like IFT88, IFT57 collects distally in cilia in fibroblasts from JATD-1 and -2 patients. Cells were stained with anti-IFT57 (green); antiacetylated α tubulin (purple); and anti-RPGRIP1L (red). Whole-field images displaying multiple cilia are available in online
supplementary figure
S8.
Similar articles
- New mutations in DYNC2H1 and WDR60 genes revealed by whole-exome sequencing in two unrelated Sardinian families with Jeune asphyxiating thoracic dystrophy.
Cossu C, Incani F, Serra ML, Coiana A, Crisponi G, Boccone L, Rosatelli MC. Cossu C, et al. Clin Chim Acta. 2016 Apr 1;455:172-80. doi: 10.1016/j.cca.2016.02.006. Epub 2016 Feb 11. Clin Chim Acta. 2016. PMID: 26874042 - Mutations in the gene encoding IFT dynein complex component WDR34 cause Jeune asphyxiating thoracic dystrophy.
Schmidts M, Vodopiutz J, Christou-Savina S, Cortés CR, McInerney-Leo AM, Emes RD, Arts HH, Tüysüz B, D'Silva J, Leo PJ, Giles TC, Oud MM, Harris JA, Koopmans M, Marshall M, Elçioglu N, Kuechler A, Bockenhauer D, Moore AT, Wilson LC, Janecke AR, Hurles ME, Emmet W, Gardiner B, Streubel B, Dopita B, Zankl A, Kayserili H, Scambler PJ, Brown MA, Beales PL, Wicking C; UK10K; Duncan EL, Mitchison HM. Schmidts M, et al. Am J Hum Genet. 2013 Nov 7;93(5):932-44. doi: 10.1016/j.ajhg.2013.10.003. Epub 2013 Oct 31. Am J Hum Genet. 2013. PMID: 24183451 Free PMC article. - Mutations in DYNC2H1, the cytoplasmic dynein 2, heavy chain 1 motor protein gene, cause short-rib polydactyly type I, Saldino-Noonan type.
Badiner N, Taylor SP, Forlenza K, Lachman RS; University of Washington Center for Mendelian Genomics; Bamshad M, Nickerson D, Cohn DH, Krakow D. Badiner N, et al. Clin Genet. 2017 Aug;92(2):158-165. doi: 10.1111/cge.12947. Epub 2017 Mar 13. Clin Genet. 2017. PMID: 27925158 Free PMC article. - Exome sequencing for the differential diagnosis of ciliary chondrodysplasias: Example of a WDR35 mutation case and review of the literature.
Antony D, Nampoory N, Bacchelli C, Melhem M, Wu K, James CT, Beales PL, Hubank M, Thomas D, Mashankar A, Behbehani K, Schmidts M, Alsmadi O. Antony D, et al. Eur J Med Genet. 2017 Dec;60(12):658-666. doi: 10.1016/j.ejmg.2017.08.019. Epub 2017 Sep 12. Eur J Med Genet. 2017. PMID: 28870638 Review. - Review: Cytoplasmic dynein motors in photoreceptors.
Dahl TM, Baehr W. Dahl TM, et al. Mol Vis. 2021 Sep 1;27:506-517. eCollection 2021. Mol Vis. 2021. PMID: 34526758 Free PMC article. Review.
Cited by
- DYNC2LI1 mutations broaden the clinical spectrum of dynein-2 defects.
Kessler K, Wunderlich I, Uebe S, Falk NS, Gießl A, Brandstätter JH, Popp B, Klinger P, Ekici AB, Sticht H, Dörr HG, Reis A, Roepman R, Seemanová E, Thiel CT. Kessler K, et al. Sci Rep. 2015 Jul 1;5:11649. doi: 10.1038/srep11649. Sci Rep. 2015. PMID: 26130459 Free PMC article. - DYNC2H1 hypomorphic or retina-predominant variants cause nonsyndromic retinal degeneration.
Vig A, Poulter JA, Ottaviani D, Tavares E, Toropova K, Tracewska AM, Mollica A, Kang J, Kehelwathugoda O, Paton T, Maynes JT, Wheway G, Arno G; Genomics England Research Consortium; Khan KN, McKibbin M, Toomes C, Ali M, Di Scipio M, Li S, Ellingford J, Black G, Webster A, Rydzanicz M, Stawiński P, Płoski R, Vincent A, Cheetham ME, Inglehearn CF, Roberts A, Heon E. Vig A, et al. Genet Med. 2020 Dec;22(12):2041-2051. doi: 10.1038/s41436-020-0915-1. Epub 2020 Aug 5. Genet Med. 2020. PMID: 32753734 Free PMC article. - Genetic variations in the DYNC2H1 gene causing SRTD3 (short-rib thoracic dysplasia 3 with or without polydactyly).
Chen W, Li Y, Zhang J, Yuan Y, Sun D, Yuan J, Yang K, Liang Y, Guo Q. Chen W, et al. Front Genet. 2023 Apr 6;14:1125473. doi: 10.3389/fgene.2023.1125473. eCollection 2023. Front Genet. 2023. PMID: 37091781 Free PMC article. - Fetal ciliopathies: a retrospective observational single-center study.
Simonini C, Floeck A, Strizek B, Mueller A, Gembruch U, Geipel A. Simonini C, et al. Arch Gynecol Obstet. 2022 Jul;306(1):71-83. doi: 10.1007/s00404-021-06265-7. Epub 2021 Oct 1. Arch Gynecol Obstet. 2022. PMID: 34596737 Free PMC article. - The role of the dynein light intermediate chain in retrograde IFT and flagellar function in Chlamydomonas.
Reck J, Schauer AM, VanderWaal Mills K, Bower R, Tritschler D, Perrone CA, Porter ME. Reck J, et al. Mol Biol Cell. 2016 Aug 1;27(15):2404-22. doi: 10.1091/mbc.E16-03-0191. Epub 2016 Jun 1. Mol Biol Cell. 2016. PMID: 27251063 Free PMC article.
References
- Jeune M, Beraud C, Carron R. Asphyxiating thoracic dystrophy with familial characteristics. Arch Fr Pediatr 1955;12:886–91 - PubMed
- Herdman RC, Langer LO. The thoracic asphyxiant dystrophy and renal disease. Am J Dis Child 1968;116:192–201 - PubMed
- Allen AW, Jr, Moon JB, Hovland KR, Minckler DS. Ocular findings in thoracic-pelvic-phalangeal dystrophy. Arch Ophthalmol 1979;97:489–92 - PubMed
- Bard LA, Bard PA, Owens GW, Hall BD. Retinal involvement in thoracic-pelvic-phalangeal dystrophy. Arch Ophthalmol 1978;96:278–81 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- MR/K000608/1/MRC_/Medical Research Council/United Kingdom
- NF-SI-0510-10268/DH_/Department of Health/United Kingdom
- WT091310/WT_/Wellcome Trust/United Kingdom
- 090532/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases