Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis - PubMed (original) (raw)
Review
Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis
Mitchell R McGill et al. Pharm Res. 2013 Sep.
Abstract
Acetaminophen (APAP) is one of the most widely used drugs. Though safe at therapeutic doses, overdose causes mitochondrial dysfunction and centrilobular necrosis in the liver. The first studies of APAP metabolism and activation were published more than 40 years ago. Most of the drug is eliminated by glucuronidation and sulfation. These reactions are catalyzed by UDP-glucuronosyltransferases (UGT1A1 and 1A6) and sulfotransferases (SULT1A1, 1A3/4, and 1E1), respectively. However, some is converted by CYP2E1 and other cytochrome P450 enzymes to a reactive intermediate that can bind to sulfhydryl groups. The metabolite can deplete liver glutathione (GSH) and modify cellular proteins. GSH binding occurs spontaneously, but may also involve GSH-S-transferases. Protein binding leads to oxidative stress and mitochondrial damage. The glucuronide, sulfate, and GSH conjugates are excreted by transporters in the canalicular (Mrp2 and Bcrp) and basolateral (Mrp3 and Mrp4) hepatocyte membranes. Conditions that interfere with metabolism and metabolic activation can alter the hepatotoxicity of the drug. Recent data providing novel insights into these processes, particularly in humans, are reviewed in the context of earlier work, and the effects of altered metabolism and reactive metabolite formation are discussed. Recent advances in the diagnostic use of serum adducts are covered.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Figures
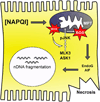
Figure 1
Late mechanisms in APAP hepatotoxicity. Protein binding, particularly to mitochondrial proteins, leads to oxidative stress and mitochondrial dysfunction. The initial oxidative stress activates the mitogen-activated protein kinases MLK3 and ASK1, which activate JNK in turn. JNK translocates to into the mitochondria, amplifying the oxidative stress and injury. Occurrence of the mitochondrial permeability transition (MPT) and rupture of the outer membrane result in release of the endonucleases apoptosis-inducing factor (AIF) and endonuclease G (EndoG), which enter the nucleus and degrade nuclear DNA.
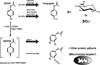
Figure 2
Metabolism and metabolic activation of APAP. Most of the drug is glucuronidated or sulfated before excretion, catalyzed by UDP-glucuronosyltransferases (UGT) and sulfotransferases (SULT), respectively. A small percentage is converted to a reactive metabolite (NAPQI) by cytochromes P450 (primarily CYP2E1). This may be regulated in part by nuclear receptors such as CAR, PXR, and RXR. The metabolite can be detoxified by conjugation with glutathione (GSH). Alternatively, it can react with protein thiols. There is evidence that mitochondrial proteins in particular are targeted by NAPQI.
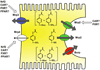
Figure 3
The glucuronide, sulfate, and glutathione conjugates of APAP are excreted into blood and bile by transporters in the basolateral and canalicular membranes, respectively. Expression of these transporters may be regulated in part by several nuclear receptors, including Nrf2, CAR, PXR, FXR, and PPAR (see text for details).
Similar articles
- A computational model for hepatotoxicity by coupling drug transport and acetaminophen metabolism equations.
Franiatte S, Clarke R, Ho H. Franiatte S, et al. Int J Numer Method Biomed Eng. 2019 Sep;35(9):e3234. doi: 10.1002/cnm.3234. Epub 2019 Jul 28. Int J Numer Method Biomed Eng. 2019. PMID: 31254976 - Enhancement of acetaminophen-induced chronic hepatotoxicity in restricted fed rats: a nonclinical approach to acetaminophen-induced chronic hepatotoxicity in susceptible patients.
Kondo K, Yamada N, Suzuki Y, Toyoda K, Hashimoto T, Takahashi A, Kobayashi A, Shoda T, Kuno H, Sugai S. Kondo K, et al. J Toxicol Sci. 2012;37(5):911-29. doi: 10.2131/jts.37.911. J Toxicol Sci. 2012. PMID: 23038001 - Role of CYP1A2 in the hepatotoxicity of acetaminophen: investigations using Cyp1a2 null mice.
Tonge RP, Kelly EJ, Bruschi SA, Kalhorn T, Eaton DL, Nebert DW, Nelson SD. Tonge RP, et al. Toxicol Appl Pharmacol. 1998 Nov;153(1):102-8. doi: 10.1006/taap.1998.8543. Toxicol Appl Pharmacol. 1998. PMID: 9875304 - Metabolic phenotyping applied to pre-clinical and clinical studies of acetaminophen metabolism and hepatotoxicity.
Coen M. Coen M. Drug Metab Rev. 2015 Feb;47(1):29-44. doi: 10.3109/03602532.2014.982865. Epub 2014 Dec 23. Drug Metab Rev. 2015. PMID: 25533740 Review. - Autophagy and acetaminophen-induced hepatotoxicity.
Shan S, Shen Z, Song F. Shan S, et al. Arch Toxicol. 2018 Jul;92(7):2153-2161. doi: 10.1007/s00204-018-2237-5. Epub 2018 Jun 6. Arch Toxicol. 2018. PMID: 29876591 Review.
Cited by
- Acetaminophen Hepatotoxicity: Paradigm for Understanding Mechanisms of Drug-Induced Liver Injury.
Jaeschke H, Ramachandran A. Jaeschke H, et al. Annu Rev Pathol. 2024 Jan 24;19:453-478. doi: 10.1146/annurev-pathmechdis-051122-094016. Annu Rev Pathol. 2024. PMID: 38265880 Free PMC article. Review. - AIM2 regulates autophagy to mitigate oxidative stress in aged mice with acute liver injury.
Hu C, Li M, Chen Y, Cheng W, Wang H, Zhou Y, Teng F, Ling T, Pan J, Xu H, Zheng Y, Ji G, Zhao T, You Q. Hu C, et al. Cell Death Discov. 2024 Mar 1;10(1):107. doi: 10.1038/s41420-024-01870-2. Cell Death Discov. 2024. PMID: 38429284 Free PMC article. - Disruption of peroxisome proliferator-activated receptor α in hepatocytes protects against acetaminophen-induced liver injury by activating the IL-6/STAT3 pathway.
Zhang Z, Yao T, Zhao N, Liu H, Cheng H, Gonzalez FJ, Wang H, Wang G, Qu A, Wang Y. Zhang Z, et al. Int J Biol Sci. 2022 Mar 6;18(6):2317-2328. doi: 10.7150/ijbs.69609. eCollection 2022. Int J Biol Sci. 2022. PMID: 35414769 Free PMC article. - Donor Hepatectomy Surgery using Ketamine to Compliment Analgesia and Reduce Morbidity - a Retrospective Chart Review Investigation.
Halaszynski TM, Dai F, Huang Y. Halaszynski TM, et al. Turk J Anaesthesiol Reanim. 2018 Feb;46(1):28-37. doi: 10.5152/TJAR.2017.33239. Epub 2017 Nov 29. Turk J Anaesthesiol Reanim. 2018. PMID: 30140498 Free PMC article. - Human multidrug resistance protein 4 (MRP4) is a cellular efflux transporter for paracetamol glutathione and cysteine conjugates.
Koenderink JB, van den Heuvel JJMW, Bilos A, Vredenburg G, Vermeulen NPE, Russel FGM. Koenderink JB, et al. Arch Toxicol. 2020 Sep;94(9):3027-3032. doi: 10.1007/s00204-020-02793-4. Epub 2020 May 29. Arch Toxicol. 2020. PMID: 32472168 Free PMC article.
References
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. - PubMed
- Krenzelok EP. The FDA Acetaminophen Advisory Committee Meeting – what is the future of acetaminophen in the United States? The perspective of a committee member. Clin Toxicol (Phila) 2009;47:784–789. - PubMed
- Bernal W. Changing patterns of causation and the use of transplantation in the United States. Semin Liv Dis. 2003;23:227–237. - PubMed
- Gow PJ, Jones RM, Dobson JL, Angus PW. Etiology and outcome of fulminant hepatic failure managed at an Australian transplant unit. J Gastroenterol Hepatol. 2004;19:154–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK070195/DK/NIDDK NIH HHS/United States
- R56 AA012916/AA/NIAAA NIH HHS/United States
- 5P20RR021940-07/RR/NCRR NIH HHS/United States
- P20 RR021940/RR/NCRR NIH HHS/United States
- 8 P20 GM103549-07/GM/NIGMS NIH HHS/United States
- T32 ES007079-26A2/ES/NIEHS NIH HHS/United States
- AA12916/AA/NIAAA NIH HHS/United States
- P20 GM103549/GM/NIGMS NIH HHS/United States
- DK070195/DK/NIDDK NIH HHS/United States
- T32 ES007079/ES/NIEHS NIH HHS/United States
- R01 AA012916/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical