Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability - PubMed (original) (raw)
Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability
Laura A Merriam et al. J Neurosci. 2013.
Abstract
After G-protein-coupled receptor activation and signaling at the plasma membrane, the receptor complex is often rapidly internalized via endocytic vesicles for trafficking into various intracellular compartments and pathways. The formation of signaling endosomes is recognized as a mechanism that produces sustained intracellular signals that may be distinct from those generated at the cell surface for cellular responses including growth, differentiation, and survival. Pituitary adenylate cyclase activating polypeptide (PACAP; Adcyap1) is a potent neurotransmitter/neurotrophic peptide and mediates its diverse cellular functions in part through internalization of its cognate G-protein-coupled PAC1 receptor (PAC1R; Adcyap1r1). In the present study, we examined whether PAC1R endocytosis participates in the regulation of neuronal excitability. Although PACAP increased excitability in 90% of guinea pig cardiac neurons, pretreatment with Pitstop 2 or dynasore to inhibit clathrin and dynamin I/II, respectively, suppressed the PACAP effect. Subsequent addition of inhibitor after the PACAP-induced increase in excitability developed gradually attenuated excitability with no changes in action potential properties. Likewise, the PACAP-induced increase in excitability was markedly decreased at ambient temperature. Receptor trafficking studies with GFP-PAC1 cell lines demonstrated the efficacy of Pitstop 2, dynasore, and low temperatures at suppressing PAC1R endocytosis. In contrast, brefeldin A pretreatments to disrupt Golgi vesicle trafficking did not blunt the PACAP effect, and PACAP/PAC1R signaling still increased neuronal cAMP production even with endocytic blockade. Our results demonstrate that PACAP/PAC1R complex endocytosis is a key step for the PACAP modulation of cardiac neuron excitability.
Figures
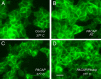
Figure 1.
Pitstop 2 and room temperature (RT) block PAC1R endocytosis. A, GFP-PAC1Rs were expressed predominantly on the cell surface of transfected, untreated control HEK 293 cells; few intracellular GFP-PAC1R–containing vesicles were evident at 37°C or room temperature (data not shown). C, The rapid internalization of GFP-PAC1Rs from the cell surface into numerous endocytic vesicles was evident after 25 n
m
PACAP addition (20 min). In contrast, maintaining the cultures at ambient room temperature (22°C; B) or pretreatment of cells with 20 μ
m
Pitstop 2 (10 min; D) followed by 25 n
m
PACAP addition (20 min) blocked GFP-PAC1R endocytosis. In both instances, the GFP-PAC1R fluorescence largely remained on the plasma membrane. Scale bar: (in D) A–D, 20 μm.
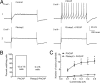
Figure 2.
Pretreatment with Pitstop 2 suppresses the PACAP-induced increase in excitability. A, Recordings from 2 different cells. A1 illustrates the increase in excitability induced by 20 n
m
PACAP. A2 shows that pretreatment with 15 μ
m
Pitstop 2 blocks the increase in excitability induced by 20 n
m
PACAP. For all recordings, a 1 s, 0.4 nA depolarizing current step was used to initiate action potential activity. B, The percentage of cells exhibiting multiple firing when exposed to PACAP alone (n = 16) was significantly greater than when exposed to PACAP after pretreatment with 15 μ
m
Pitstop 2 (n = 8; Fisher's exact test, p < 0.0001). C, Averaged excitability curves show that Pitstop 2 greatly suppressed the PACAP-induced increase in excitability. Asterisks indicate that the number of action potentials generated at each current step was significantly greater in PACAP (n = 16) than in PACAP and Pitstop 2 (n = 8; unpaired t test, p < 0.0001 for steps 0.2–0.5 nA).
Figure 3.
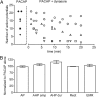
Pretreatment with dynasore suppresses the PACAP-induced increase in excitability. A, The percentage of cells exhibiting multiple firing when exposed to PACAP alone (n = 13) was significantly greater than when exposed to PACAP after pretreatment with 20 μ
m
dynasore (n = 13; Fisher's exact test, p < 0.0001). B, Averaged excitability curves show that dynasore greatly suppressed the PACAP-induced increase in excitability. Asterisks indicate that the number of action potentials generated at each current step was significantly greater in PACAP (n = 13) than in PACAP and dynasore (n = 13; unpaired t test, p = 0.0005 for 0.1 nA; p < 0.0001 for 0.2 nA; p = 0.0002 for 0.3 nA; p = 0.0001 for 0.4 nA; p = 0.0003 for 0.5 nA). C, Recordings illustrated that bethanechol could increase excitability in a cell pretreated with dynasore and exposed to PACAP. Left trace shows the phasic-firing pattern generated by a 1 s, 0.4 nA depolarizing current pulse in a cell pretreated with dynasore and exposed to PACAP. After local puffer application of bethanechol, the same depolarizing current step elicited multiple firing.
Figure 4.
The PACAP-induced increase in excitability is temperature sensitive. A, Recordings from different cells showed that 20 n
m
PACAP increased excitability when the bath solution was 33°C (A1), but not when the bath temperature was 22°C (A2). The recording in A3 demonstrated that the addition of 1 m
m
BaCl2 (Ba2+) increased excitability at 22°C. In all three recordings, the cells exhibited a phasic-firing pattern before the addition of PACAP (A1 and A2) or barium (A3). The firing pattern shifted to multiple firing in A1 and A3, but not in A2. The amplitude of the 1 s depolarizing current pulse was 0.3 nA in each experiment. B, The percentage of cells exhibiting multiple firing in 20 n
m
PACAP was significantly greater when the temperature was 32–34°C (n = 13 cells) than when the bath temperature was 21–25°C (n = 23 cells; Fisher's exact test, p < 0.0001). At 32–34°C: control (n = 28) versus PACAP (n = 13), significantly different, p < 0.0001; at 21–25°C: control (n = 10) versus PACAP (n = 23), not significant, p = 0.5363). C, Averaged excitability curves generated in the cells maintained at either 33–34°C or 21–25°C before and during exposure to 20 n
m
PACAP. The number of action potentials generated at each current step was significantly greater (indicated by asterisks) at the warmer temperature in the presence of PACAP (n = 13) compared with control at warm temperature (n = 28; unpaired t test, p = 0.0011 at 0.1 nA; p = 0.0002 at 0.2 nA; p = 0.0004 at 0.3 nA; p = 0.0002 at 0.4 nA; p = 0.0005 at 0.5 nA), and to PACAP at room temperature (n = 23; unpaired t test, p = 0.0029 at 0.1 nA; p = 0.0003 at 0.2 nA; p = 0.0005 at 0.3 nA; p = 0.0003 at 0.4 nA; p = 0.0005 at 0.5 nA). The number of action potentials generated at each current step was not different between control (n = 10) and PACAP (n = 23) at room temperature (p = 0.3502 at 0.1 nA; p = 0.4040 at 0.2 nA; p = 0.7132 at 0.3 nA; p = 0.4495 at 0.4 nA; p = 0.6642 at 0.5 nA).
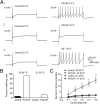
Figure 5.
Pitstop 2 progressively reverses the PACAP effect after the increase in excitability has developed. A, Results from 6 different cells in which 15 μ
m
Pitstop 2 was added after the PACAP-induced shift from a phasic- to a multiple-firing pattern had developed. Note that the number of action potentials elicited by the same stimulus (1 s, 0.5 nA for the cell indicated by the solid and open circles; 1 s, 0.4 nA for the other cells) declined until action potential generation reverted back to a phasic-firing pattern in 5 of the 6 cells. In the sixth cell, the number of action potentials declined progressively until the impalement was lost. Each solid symbol represents data from one cell recorded in PACAP alone, followed in time by the corresponding open symbol representing the data recorded from the same cell after Pitstop 2 was applied. B, Normalized data from the 5 cells that had returned to a phasic-firing pattern, which illustrated that the action potential amplitude (AP), AHP amplitude (AHP amp), AHP duration (AHP dur), the rectification occurring with hyperpolarizing steps (Rect), and effective membrane resistance (EMR) were not altered by Pitstop 2 at a time when the firing pattern was phasic (n = 5; paired t test, AP: p = 0.2104; AHP: p = 0.2359; AHP dur: p = 0.0882; Rect: p = 0.4792; EMR: p = 0.6553). The results presented in B are normalized to values obtained in the presence of PACAP before the addition of Pitstop 2.
Figure 6.
Dynasore reduces the PACAP effect after the increase in excitability have developed. A, Results from 6 different cells exposed to 20 μ
m
dynasore after the PACAP-induced shift from a phasic- to a multiple-firing pattern had developed. Each solid symbol represents data from one cell recorded in PACAP alone, followed in time by the corresponding open symbol representing the data recorded from the same cell after dynasore was applied. Note that the number of action potentials elicited by the same stimulus (1 s, 0.4 nA) declined over time in dynasore. In two cells, action potential generation reverted back to a phasic-firing pattern. In three other cells, although action potential generation declined, the firing pattern remained multiple at the time the recording was terminated. In the remaining cell, the firing pattern changed to a lesser extent in dynasore. B, Normalized data from 5 cells illustrating that the action potential amplitude (AP), AHP amplitude (AHP amp), AHP duration (AHP dur), the rectification occurring with hyperpolarizing steps (Rect, and effective membrane resistance (EMR) was not altered by exposure to dynasore for 10–20 min (n = 5 paired t test, AP: p = 0.5957; AHP: p = 0.1863; AHP dur: p = 0.0573; Rect: p = 0.3437; EMR: p = 0.8442). The results presented in B are normalized to values obtained in the presence of PACAP before the addition of dynasore.
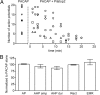
Figure 7.
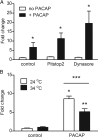
PACAP stimulates cAMP generation in dissociated rat neonatal SCG neurons after pretreatment with Pitstop 2 or dynasore and when cells are maintained at room temperature. A, PACAP increased cAMP production at 34°C after pretreatment with Pitstop 2 (10 min, 15 μ
m
) or dynasore (10 min, 20 μ
m
) and under control conditions. Values are expressed as the fold change of femtomoles/well determined at 34°C. Asterisks indicate that the generation of cAMP by PACAP at 34°C in inhibitor-pretreated cells was significantly greater than with cells exposed to inhibitor without PACAP (unpaired t test; n = 4 in all cases; control vs PACAP, p = 0.0025; dynasore vs PACAP + dynasore, p = 0.0144; Pitstop 2 vs PACAP + Pitstop 2, p = 0.0058). B, At 24°C, PACAP (n = 3) significantly increased the generation of cAMP compared with control (n = 3). *p = 0.0004, unpaired t test. At 34°C, PACAP (n = 3) significantly increased the generation of cAMP compared with control (n = 3). **p = 0.0005, unpaired t test. The generation of cAMP was significantly greater at 24°C (n = 3) than at 34°C (n = 3) after a 30 min incubation in 20 n
m
PACAP. ***p = 0.0315, unpaired t test. Values are expressed as the fold change of femtomoles/well determined at 34°C.
Similar articles
- PACAP-Induced PAC1 Receptor Internalization and Recruitment of Endosomal Signaling Regulate Cardiac Neuron Excitability.
Parsons RL, May V. Parsons RL, et al. J Mol Neurosci. 2019 Jul;68(3):340-347. doi: 10.1007/s12031-018-1127-x. Epub 2018 Jul 27. J Mol Neurosci. 2019. PMID: 30054797 Free PMC article. Review. - Src family kinase inhibitors blunt PACAP-induced PAC1 receptor endocytosis, phosphorylation of ERK, and the increase in cardiac neuron excitability.
Tompkins JD, Clason TA, Buttolph TR, Girard BM, Linden AK, Hardwick JC, Merriam LA, May V, Parsons RL. Tompkins JD, et al. Am J Physiol Cell Physiol. 2018 Feb 1;314(2):C233-C241. doi: 10.1152/ajpcell.00223.2017. Epub 2017 Nov 15. Am J Physiol Cell Physiol. 2018. PMID: 29141923 Free PMC article. - PAC1 Receptor Internalization and Endosomal MEK/ERK Activation Is Essential for PACAP-Mediated Neuronal Excitability.
May V, Johnson GC, Hammack SE, Braas KM, Parsons RL. May V, et al. J Mol Neurosci. 2021 Aug;71(8):1536-1542. doi: 10.1007/s12031-021-01821-x. Epub 2021 Mar 6. J Mol Neurosci. 2021. PMID: 33675454 Free PMC article. Review. - Recruitment of endosomal signaling mediates the forskolin modulation of guinea pig cardiac neuron excitability.
Hardwick JC, Clason TA, Tompkins JD, Girard BM, Baran CN, Merriam LA, May V, Parsons RL. Hardwick JC, et al. Am J Physiol Cell Physiol. 2017 Aug 1;313(2):C219-C227. doi: 10.1152/ajpcell.00094.2017. Epub 2017 Jun 7. Am J Physiol Cell Physiol. 2017. PMID: 28592413 Free PMC article. - Activation of MEK/ERK signaling contributes to the PACAP-induced increase in guinea pig cardiac neuron excitability.
Tompkins JD, Clason TA, Hardwick JC, Girard BM, Merriam LA, May V, Parsons RL. Tompkins JD, et al. Am J Physiol Cell Physiol. 2016 Oct 1;311(4):C643-C651. doi: 10.1152/ajpcell.00164.2016. Epub 2016 Aug 3. Am J Physiol Cell Physiol. 2016. PMID: 27488668 Free PMC article.
Cited by
- Synthesis of the Pitstop family of clathrin inhibitors.
Robertson MJ, Deane FM, Stahlschmidt W, von Kleist L, Haucke V, Robinson PJ, McCluskey A. Robertson MJ, et al. Nat Protoc. 2014 Jul;9(7):1592-606. doi: 10.1038/nprot.2014.106. Epub 2014 Jun 12. Nat Protoc. 2014. PMID: 24922269 - Nickel suppresses the PACAP-induced increase in guinea pig cardiac neuron excitability.
Tompkins JD, Merriam LA, Girard BM, May V, Parsons RL. Tompkins JD, et al. Am J Physiol Cell Physiol. 2015 Jun 1;308(11):C857-66. doi: 10.1152/ajpcell.00403.2014. Epub 2015 Mar 25. Am J Physiol Cell Physiol. 2015. PMID: 25810261 Free PMC article. - EGFR-dependent endocytosis of Wnt9a and Fzd9b promotes β-catenin signaling during hematopoietic stem cell development in zebrafish.
Nguyen N, Carpenter KA, Ensing J, Gilliland C, Rudisel EJ, Mu EM, Thurlow KE, Triche TJ Jr, Grainger S. Nguyen N, et al. Sci Signal. 2024 Apr 16;17(832):eadf4299. doi: 10.1126/scisignal.adf4299. Epub 2024 Apr 16. Sci Signal. 2024. PMID: 38626007 - Endocrinology and the brain: corticotropin-releasing hormone signaling.
Inda C, Armando NG, Dos Santos Claro PA, Silberstein S. Inda C, et al. Endocr Connect. 2017 Aug;6(6):R99-R120. doi: 10.1530/EC-17-0111. Epub 2017 Jul 14. Endocr Connect. 2017. PMID: 28710078 Free PMC article. Review. - Luminescence-activated nucleotide cyclase regulates spatial and temporal cAMP synthesis.
Naim N, White AD, Reece JM, Wankhede M, Zhang X, Vilardaga JP, Altschuler DL. Naim N, et al. J Biol Chem. 2019 Jan 25;294(4):1095-1103. doi: 10.1074/jbc.AC118.004905. Epub 2018 Dec 17. J Biol Chem. 2019. PMID: 30559293 Free PMC article.
References
- Adams DJ, Cuevas J. Electrophysiological properties of intrinsic cardiac neurons. In: Armour JA, Ardell JL, editors. Basic and clinical neurocardiology. Oxford: Oxford UP; 2004. pp. 1–60.
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spacially resolved dynamics of CAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. - PubMed
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. - PubMed
- Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC1 receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274:27702–27710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL098589/HL/NHLBI NIH HHS/United States
- P30 GM103498/GM/NIGMS NIH HHS/United States
- P20 RR016435/RR/NCRR NIH HHS/United States
- P20 RR16435/RR/NCRR NIH HHS/United States
- HL098589/HL/NHLBI NIH HHS/United States
- P30 RR032135/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources