Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b - PubMed (original) (raw)
Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b
Margaret E Ackerman et al. J Virol. 2013 May.
Abstract
While development of an HIV vaccine that can induce neutralizing antibodies remains a priority, decades of research have proven that this is a daunting task. However, accumulating evidence suggests that antibodies with the capacity to harness innate immunity may provide some protection. While significant research has focused on the cytolytic properties of antibodies in acquisition and control, less is known about the role of additional effector functions. In this study, we investigated antibody-dependent phagocytosis of HIV immune complexes, and we observed significant differences in the ability of antibodies from infected subjects to mediate this critical effector function. We observed both quantitative differences in the capacity of antibodies to drive phagocytosis and qualitative differences in their FcγR usage profile. We demonstrate that antibodies from controllers and untreated progressors exhibit increased phagocytic activity, altered Fc domain glycosylation, and skewed interactions with FcγR2a and FcγR2b in both bulk plasma and HIV-specific IgG. While increased phagocytic activity may directly influence immune activation via clearance of inflammatory immune complexes, it is also plausible that Fc receptor usage patterns may regulate the immune response by modulating downstream signals following phagocytosis--driving passive degradation of internalized virus, release of immune modulating cytokines and chemokines, or priming of a more effective adaptive immune response.
Figures
Fig 1
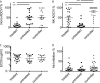
Differential effector function of antibodies from HIV-positive subjects. (A and B) ADCVI activities of antibodies from different subject groups in assays utilizing primary monocytes (A) or NK cells (B) as effectors. (C) Binding titers (EC50, in μg/ml) of anti-envelope antibodies. (D) Antibody neutralization as determined by viral inhibition in the absence of effector cells. **, P < 0.005; ***, P < 0.0005.
Fig 2
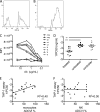
Differential phagocytic function measured at high throughput. (A and B) Representative histograms of bead uptake by THP-1 cells for antibody samples with low (A) and high (B) phagocytosis activity. (C) Representative phagocytosis dose-response curves for HIV-negative subjects (neg), controllers (ctr), untreated subjects (un), and treated subjects (tx). Phagocytic activity is presented as iMFI. (D) Phagocytic potency of antibodies from HIV-infected subjects. Reciprocal log PC50s (1/concentration at which half-maximal phagocytosis was observed) for gp120-coated bead uptake by a monocytic cell line are shown. (E and F) Correlation of viral inhibition in primary monocytes (E) or NK cells (F) with phagocytic potential (1/PC50) determined by the THP-1 phagocytosis assay. **, P < 0.005; ***, P < 0.0005.
Fig 3
Phagocytic dependence on specific FcγR. (A) Phagocytosis by cells pretreated with FcγR2a-, FcγR2b-, and FcγR3a-blocking antibodies or left untreated was determined. The ratio of bead uptake under blocked and unblocked conditions is presented. The effect of blocking FcγR3a (B), FcγR2a (C), and FcγR2b (D) was determined within each subject class, exposing differences between patient groups in reliance on these receptors. *, P < 0.05; ***, P < 0.0005.
Fig 4
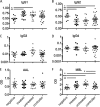
Plasma IgG exhibits differential phagocytosis activity among subject groups. (A to C) Representative flow cytometric dot plot (A) and histograms (B and C) of competitive uptake of green fluorescent beads (B) coated with bulk antibody from a low-activity subject relative to uptake of red fluorescent beads (C) opsonized with bulk antibody from a high-activity subject. (D and E) Beads opsonized with bulk antibody from negative subjects were competed against other HIV-negative subjects (Neg), chronically infected subjects (Chr), or controllers (Ctr) (D) or all other subjects (E). Data presented are ratios of phagocytosis of red to green beads. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Fig 5
Plasma antibody subclass and glycosylation. (A to D) Fraction of plasma IgG of the IgG1 (A), IgG2 (B), IgG3 (C), and IgG4 (D) subclasses. (E and F) Plasma IgG glycosylation as determined by ELISA for fucose (E) or terminal mannose (F). OD, optical density. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Fig 6
Differential phagocytosis is associated with binding to FcγR2. (A) Ratio of binding to the activating FcγR2a relative to the inhibitory FcγR2b as determined by ELISA. (B) Correlation of log phagocytic potency (1/PC50) with binding to FcγR2a/FcγR2b (Biacore analysis). *, P < 0.05; **, P < 0.005.
Similar articles
- Multiplexed Fc array for evaluation of antigen-specific antibody effector profiles.
Brown EP, Dowell KG, Boesch AW, Normandin E, Mahan AE, Chu T, Barouch DH, Bailey-Kellogg C, Alter G, Ackerman ME. Brown EP, et al. J Immunol Methods. 2017 Apr;443:33-44. doi: 10.1016/j.jim.2017.01.010. Epub 2017 Feb 3. J Immunol Methods. 2017. PMID: 28163018 Free PMC article. - Anti-retroviral antibody FcγR-mediated effector functions.
Bournazos S, Ravetch JV. Bournazos S, et al. Immunol Rev. 2017 Jan;275(1):285-295. doi: 10.1111/imr.12482. Immunol Rev. 2017. PMID: 28133801 Free PMC article. Review. - Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner.
Mullarkey CE, Bailey MJ, Golubeva DA, Tan GS, Nachbagauer R, He W, Novakowski KE, Bowdish DM, Miller MS, Palese P. Mullarkey CE, et al. mBio. 2016 Oct 4;7(5):e01624-16. doi: 10.1128/mBio.01624-16. mBio. 2016. PMID: 27703076 Free PMC article. - Antigen-Specific Antibody Glycosylation Is Regulated via Vaccination.
Mahan AE, Jennewein MF, Suscovich T, Dionne K, Tedesco J, Chung AW, Streeck H, Pau M, Schuitemaker H, Francis D, Fast P, Laufer D, Walker BD, Baden L, Barouch DH, Alter G. Mahan AE, et al. PLoS Pathog. 2016 Mar 16;12(3):e1005456. doi: 10.1371/journal.ppat.1005456. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 26982805 Free PMC article. - Antibody Functional Assays as Measures of Fc Receptor-Mediated Immunity to HIV - New Technologies and their Impact on the HIV Vaccine Field.
Wines BD, Billings H, Mclean MR, Kent SJ, Hogarth PM. Wines BD, et al. Curr HIV Res. 2017;15(3):202-215. doi: 10.2174/1570162X15666170320112247. Curr HIV Res. 2017. PMID: 28322167 Free PMC article. Review.
Cited by
- Networking at the level of host immunity: immune cell interactions during persistent viral infections.
Ng CT, Snell LM, Brooks DG, Oldstone MB. Ng CT, et al. Cell Host Microbe. 2013 Jun 12;13(6):652-64. doi: 10.1016/j.chom.2013.05.014. Cell Host Microbe. 2013. PMID: 23768490 Free PMC article. Review. - Expression Profile of Human Fc Receptors in Mucosal Tissue: Implications for Antibody-Dependent Cellular Effector Functions Targeting HIV-1 Transmission.
Cheeseman HM, Carias AM, Evans AB, Olejniczak NJ, Ziprin P, King DF, Hope TJ, Shattock RJ. Cheeseman HM, et al. PLoS One. 2016 May 10;11(5):e0154656. doi: 10.1371/journal.pone.0154656. eCollection 2016. PLoS One. 2016. PMID: 27164006 Free PMC article. - Non-Neutralizing Antibodies Directed against HIV and Their Functions.
Mayr LM, Su B, Moog C. Mayr LM, et al. Front Immunol. 2017 Nov 20;8:1590. doi: 10.3389/fimmu.2017.01590. eCollection 2017. Front Immunol. 2017. PMID: 29209323 Free PMC article. Review. - Temporal variation in HIV-specific IgG subclass antibodies during acute infection differentiates spontaneous controllers from chronic progressors.
Sadanand S, Das J, Chung AW, Schoen MK, Lane S, Suscovich TJ, Streeck H, Smith DM, Little SJ, Lauffenburger DA, Richman DD, Alter G. Sadanand S, et al. AIDS. 2018 Feb 20;32(4):443-450. doi: 10.1097/QAD.0000000000001716. AIDS. 2018. PMID: 29239894 Free PMC article. - Functional Homology for Antibody-Dependent Phagocytosis Across Humans and Rhesus Macaques.
Pollara J, Tay MZ, Edwards RW, Goodman D, Crowley AR, Edwards RJ, Easterhoff D, Conley HE, Hoxie T, Gurley T, Jones C, Machiele E, Tuyishime M, Donahue E, Jha S, Spreng RL, Hope TJ, Wiehe K, He MM, Moody MA, Saunders KO, Ackerman ME, Ferrari G, Tomaras GD. Pollara J, et al. Front Immunol. 2021 May 20;12:678511. doi: 10.3389/fimmu.2021.678511. eCollection 2021. Front Immunol. 2021. PMID: 34093580 Free PMC article.
References
- Takai T. 2005. Fc receptors and their role in immune regulation and autoimmunity. J. Clin. Immunol. 25:1–18 - PubMed
- Aasa-Chapman MM, Holuigue S, Aubin K, Wong M, Jones NA, Cornforth D, Pellegrino P, Newton P, Williams I, Borrow P, McKnight A. 2005. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J. Virol. 79:2823–2830 - PMC - PubMed
- Ackerman ME, Dugast AS, Alter G. 2012. Emerging concepts on the role of innate immunity in the prevention and control of HIV infection. Annu. Rev. Med. 63:113–130 - PubMed
- Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, Ahmad A. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J. Clin. Immunol. 21:227–233 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources