Devastating decline of forest elephants in central Africa - PubMed (original) (raw)
doi: 10.1371/journal.pone.0059469. Epub 2013 Mar 4.
Samantha Strindberg, Stephen Blake, George Wittemyer, John Hart, Elizabeth A Williamson, Rostand Aba'a, Gaspard Abitsi, Ruffin D Ambahe, Fidèl Amsini, Parfait C Bakabana, Thurston Cleveland Hicks, Rosine E Bayogo, Martha Bechem, Rene L Beyers, Anicet N Bezangoye, Patrick Boundja, Nicolas Bout, Marc Ella Akou, Lambert Bene Bene, Bernard Fosso, Elizabeth Greengrass, Falk Grossmann, Clement Ikamba-Nkulu, Omari Ilambu, Bila-Isia Inogwabini, Fortune Iyenguet, Franck Kiminou, Max Kokangoye, Deo Kujirakwinja, Stephanie Latour, Innocent Liengola, Quevain Mackaya, Jacob Madidi, Bola Madzoke, Calixte Makoumbou, Guy-Aimé Malanda, Richard Malonga, Olivier Mbani, Valentin A Mbendzo, Edgar Ambassa, Albert Ekinde, Yves Mihindou, Bethan J Morgan, Prosper Motsaba, Gabin Moukala, Anselme Mounguengui, Brice S Mowawa, Christian Ndzai, Stuart Nixon, Pele Nkumu, Fabian Nzolani, Lilian Pintea, Andrew Plumptre, Hugo Rainey, Bruno Bokoto de Semboli, Adeline Serckx, Emma Stokes, Andrea Turkalo, Hilde Vanleeuwe, Ashley Vosper, Ymke Warren
Affiliations
- PMID: 23469289
- PMCID: PMC3587600
- DOI: 10.1371/journal.pone.0059469
Devastating decline of forest elephants in central Africa
Fiona Maisels et al. PLoS One. 2013.
Abstract
African forest elephants- taxonomically and functionally unique-are being poached at accelerating rates, but we lack range-wide information on the repercussions. Analysis of the largest survey dataset ever assembled for forest elephants (80 foot-surveys; covering 13,000 km; 91,600 person-days of fieldwork) revealed that population size declined by ca. 62% between 2002-2011, and the taxon lost 30% of its geographical range. The population is now less than 10% of its potential size, occupying less than 25% of its potential range. High human population density, hunting intensity, absence of law enforcement, poor governance, and proximity to expanding infrastructure are the strongest predictors of decline. To save the remaining African forest elephants, illegal poaching for ivory and encroachment into core elephant habitat must be stopped. In addition, the international demand for ivory, which fuels illegal trade, must be dramatically reduced.
Conflict of interest statement
Competing Interests: The authors have the following interest: This study was in part supported by the following: Busch Gardens and TOTAL Gabon. CIB (Congolaise Industrielle du Bois) provided some logistical help for the 2006 and 2010–11 surveys in the following northern Congo sites (Pokola, Kabo, Loundougou. This does not alter the authors’ adherence to all the PLOS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Figures
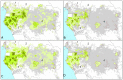
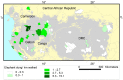
Figure 1. Elephant dung density and range reduction across the Central African forests.
Predictions are shown for (A) 2002 and (B) 2011 for the model with variables: survey year∧, Human Influence Index***, corruption*** and the presence/absence of guards***, and (C) 2002 and (D) 2011 for the model with variables: survey year∧, proximity to road∧, human population density***, corruption*** and the presence/absence of guards*** (P-values are: ‘***’ <0.001 and ‘∧’ <0.1). Increasingly darker shades of green correspond to higher densities, grey represents extremely low elephant density range (the first interval: 0–100 elephant dung piles/km2) and white is non-habitat (80 survey sites outlined in red). Cutpoints are: 0; 100; 250; 500; 1,000; 1,500; 3,000; 5,000; and 7,500 dung piles/km2. Countries 1–5 are: Cameroon; Central African Republic; Republic of Congo; DRC; Gabon.
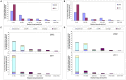
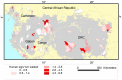
Figure 2. Estimated change in elephant dung density (/km2) distribution during 2002–2011 across the Central African forests.
Results are shown as a percentage of the total area of potential elephant habitat overall (A & B) and by country (C & D) for the predictive model with variables: (A & C) survey year, Human Influence Index, corruption and the presence/absence of guards, and (B & D) survey year, proximity to road, human population density, corruption and the presence/absence of guards. The dung density (per km2) intervals are unequal and correspond to the following elephant population categories: extremely low density (0–100), very low (100–250), low (250–500), medium (500–1,000), high (1,000–3,000) and very high (3,000–7,500). With the loss of very high elephant populations in 2011, there is a significant shift into the lower density intervals over the nine years.
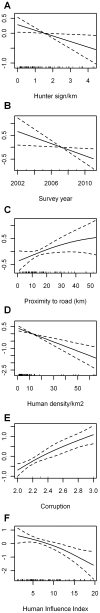
Figure 3. Estimated conditional dependence of elephant dung density for top-ranked multi-variable models including hunter sign.
Results are shown for the top-ranked model with variables: (A) hunter sign*, (B) survey year*, (C) proximity to roads∧, (D) human population density***, (E) corruption*** (higher values = less corrupt) and presence/absence of guards***. Also shown is (F) the Human Influence Index (HII) for the model with proximity to road and human population density variables replaced by the HII, i.e. one of the top-ranking models with variables: hunter sign**, survey year*, HII*, corruption***, and presence/absence of guards***. P-value significance codes are: ‘***’<0.001, ‘**’<0.01, ‘*’<0.05, and ‘∧’<0.1. Plot components are: Estimates on the scale of the linear predictor (solid lines) with the y-axis scale for each variable selected to optimally display the results, confidence intervals (dashed lines), and explanatory variable values of observations with a focus on the core 95% of values for hunter sign, proximity to road and human population density (rug plot - short vertical bars along each x-axis showing the x value for each site).
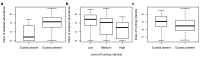
Figure 4. Boxplots of indices of elephant abundance and hunting intensity.
Summaries shown are the natural logarithm of: (A) elephant dung encounter rate per 100 km grouped by the presence/absence of wildlife guards, (B) elephant dung encounter rate per 100 km grouped by the level of hunting intensity (group cutpoints are 0.6 and 1.75 hunter sign/km), and (C) hunter-sign frequency per 100 km grouped by the presence/absence of wildlife guards. Box-widths are proportional to the number of observations in each group.
Figure 5. Encounter rate of elephant dung per kilometre.
Results are shown for the 80 survey sites in Central Africa included in this study. Grey shading represents forest cover.
Figure 6. Encounter rate of hunter sign per kilometre.
Results are shown for the 80 survey sites in Central Africa included in this study. Grey shading represents forest cover.
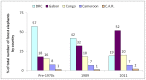
Figure 7. Percentage breakdown of the total number of forest elephants by country.
Results are shown for 3 time periods: pre-1970s and 1989 and 2011 (this study).
Similar articles
- Drivers and facilitators of the illegal killing of elephants across 64 African sites.
Kuiper T, Altwegg R, Beale C, Carroll T, Dublin HT, Hauenstein S, Kshatriya M, Schwarz C, Thouless CR, Royle A, Milner-Gulland EJ. Kuiper T, et al. Proc Biol Sci. 2023 Jan 11;290(1990):20222270. doi: 10.1098/rspb.2022.2270. Epub 2023 Jan 11. Proc Biol Sci. 2023. PMID: 36629103 Free PMC article. - The consequences of poaching and anthropogenic change for forest elephants.
Breuer T, Maisels F, Fishlock V. Breuer T, et al. Conserv Biol. 2016 Oct;30(5):1019-26. doi: 10.1111/cobi.12679. Epub 2016 Apr 7. Conserv Biol. 2016. PMID: 26801000 - Estimating economic losses to tourism in Africa from the illegal killing of elephants.
Naidoo R, Fisher B, Manica A, Balmford A. Naidoo R, et al. Nat Commun. 2016 Nov 1;7:13379. doi: 10.1038/ncomms13379. Nat Commun. 2016. PMID: 27802262 Free PMC article. - Forest elephant crisis in the Congo Basin.
Blake S, Strindberg S, Boudjan P, Makombo C, Bila-Isia I, Ilambu O, Grossmann F, Bene-Bene L, de Semboli B, Mbenzo V, S'hwa D, Bayogo R, Williamson L, Fay M, Hart J, Maisels F. Blake S, et al. PLoS Biol. 2007 Apr;5(4):e111. doi: 10.1371/journal.pbio.0050111. PLoS Biol. 2007. PMID: 17407383 Free PMC article. - Elephant behaviour and conservation: social relationships, the effects of poaching, and genetic tools for management.
Archie EA, Chiyo PI. Archie EA, et al. Mol Ecol. 2012 Feb;21(3):765-78. doi: 10.1111/j.1365-294X.2011.05237.x. Epub 2011 Aug 31. Mol Ecol. 2012. PMID: 21880086 Review.
Cited by
- FSC-certified forest management benefits large mammals compared to non-FSC.
Zwerts JA, Sterck EHM, Verweij PA, Maisels F, van der Waarde J, Geelen EAM, Tchoumba GB, Donfouet Zebaze HF, van Kuijk M. Zwerts JA, et al. Nature. 2024 Apr;628(8008):563-568. doi: 10.1038/s41586-024-07257-8. Epub 2024 Apr 10. Nature. 2024. PMID: 38600379 Free PMC article. - Extending the conservation impact of great ape research: Flagship species sites facilitate biodiversity assessments and land preservation.
Morgan D, Strindberg S, McElmurray P, Zambarda A, Singono I, Huskisson S, Musgrave S, Ayina CE, Funkhouser J, Hellmuth H, Joshi P, Cassidy R, Sanz C. Morgan D, et al. Primates. 2024 Nov;65(6):571-591. doi: 10.1007/s10329-023-01080-x. Epub 2023 Sep 8. Primates. 2024. PMID: 37682371 - To avoid carbon degradation in tropical forests, conserve wildlife.
Bennett EL, Robinson JG. Bennett EL, et al. PLoS Biol. 2023 Aug 29;21(8):e3002262. doi: 10.1371/journal.pbio.3002262. eCollection 2023 Aug. PLoS Biol. 2023. PMID: 37643160 Free PMC article. - Trenches reduce crop foraging by elephants: Lessons from Kibale National Park, Uganda for elephant conservation in densely settled rural landscapes.
Rogers A, Treves A, Karamagi R, Nyakoojo M, Naughton-Treves L. Rogers A, et al. PLoS One. 2023 Jul 26;18(7):e0288115. doi: 10.1371/journal.pone.0288115. eCollection 2023. PLoS One. 2023. PMID: 37494325 Free PMC article. - Determining the Provenance of Traded Wildlife in the Philippines.
Brandis KJ, Meagher P, Schoppe S, Zawada K, Widmann I, Widmann P, Dolorosa RG, Francis R. Brandis KJ, et al. Animals (Basel). 2023 Jun 30;13(13):2165. doi: 10.3390/ani13132165. Animals (Basel). 2023. PMID: 37443963 Free PMC article.
References
- IUCN/SSC (2008) Strategic planning for species conservation- a handbook. The species conservation planning task force. Species Survival Commission, IUCN. Gland, Switzerland: IUCN Species Survival Commission. 104. Available: http://data.iucn.org/dbtw-wpd/edocs/2008-047.pdf. Accessed 2013 Feb 20.
- IUCN (2012) IUCN Red List of threatened species. Version 2012.2. Cambridge, UK: IUCN. Available: http://www.iucnredlist.org/. Accessed 2013 Feb 20.
- AfESG (2003) Statement on the taxonomy of extant Loxodonta, December 2003. IUCN. 2. Available: www.iucnredlist.org/documents/AfESGGeneticStatement.pdf. Accessed 2013 Feb 20.
- Blanc J (2008) Loxodonta africana IUCN Available: http://www.iucnredlist.org/documents/attach/12392.pdf. Accessed 2013 Feb 20.
- Grubb P, Groves CP, Dudley JP, Shoshani J (2000) Living African elephants belong to two species: Loxodonta africana (Blumenbach, 1797) and Loxodonta cyclotis (Matschie, 1900). Elephant 2: 1–4.
Publication types
MeSH terms
Grants and funding
For funding of the survey work the authors thank (in alphabetical order) Nancy Abraham, the African Wildlife Foundation, Beneficia Foundation, Busch Gardens, Columbus Zoo, Conservation International, Daniel K. Thorne Foundation, Diane Fossey Gorilla Foundation International, Espèces Phares (European Union), Ecosystèmes Forestiers d’Afrique Centrale (ECOFAC), Fauna and Flora International, Frankfurt Zoological Society, IUCN Netherlands, John D. and Catherine T. MacArthur Foundation, KFW, LifeWeb (Spain), National Fund for Scientific Research (FNRS, Belgium), Offield Family Foundation, Operation Loango, Prince Bernhard Wildlife Fund, RAPAC, The Arcus Foundation, The Aspinall Foundation, The Born Free Foundation, The Institute for Biodiversity and Ecosystem Dynamics at The University of Amsterdam, The Jane Goodall Institute, The Liz Claiborne and Art Ortenberg Foundation, The Lucie Burgers Foundation, The Wasmoeth Wildlife Foundation and Karl Ammann, Total Gabon, United States Agency for International Development (USAID CARPE), USFWS Great Ape Conservation Fund, USFWS African Elephant Conservation Fund, Wildlife Conservation Society, World Wildlife Fund and the Zoological Society of London. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources