Serum-starved adipose-derived stromal cells ameliorate crescentic GN by promoting immunoregulatory macrophages - PubMed (original) (raw)
Serum-starved adipose-derived stromal cells ameliorate crescentic GN by promoting immunoregulatory macrophages
Kazuhiro Furuhashi et al. J Am Soc Nephrol. 2013 Mar.
Abstract
Mesenchymal stromal cells (MSCs) derived from adipose tissue have immunomodulatory effects, suggesting that they may have therapeutic potential for crescentic GN. Here, we systemically administered adipose-derived stromal cells (ASCs) in a rat model of anti-glomerular basement membrane (anti-GBM) disease and found that this treatment protected against renal injury and decreased proteinuria, crescent formation, and infiltration by glomerular leukocytes, including neutrophils, CD8(+) T cells, and CD68(+) macrophages. Interestingly, ASCs cultured under low-serum conditions (LASCs), but not bone marrow-derived MSCs (BM-MSCs), increased the number of immunoregulatory CD163(+) macrophages in diseased glomeruli. Macrophages cocultured with ASCs, but not with BM-MSCs, adopted an immunoregulatory phenotype. Notably, LASCs polarized macrophages into CD163(+) immunoregulatory cells associated with IL-10 production more efficiently than ASCs cultured under high-serum conditions. Pharmaceutical ablation of PGE2 production, blocking the EP4 receptor, or neutralizing IL-6 in the coculture medium all significantly reversed this LASC-induced conversion of macrophages. Furthermore, pretreating LASCs with aspirin or cyclooxygenase-2 inhibitors impaired the ability of LASCs to ameliorate nephritogenic IgG-mediated renal injury. Taken together, these results suggest that LASCs exert renoprotective effects in anti-GBM GN by promoting the phenotypic conversion of macrophages to immunoregulatory cells, suggesting that LASC transfer may represent a therapeutic strategy for crescentic GN.
Figures
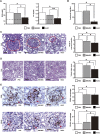
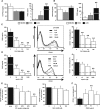
Figure 1.
Systemic administration of BM-MSCs and HASCs has a protective effect against rat anti-GBM GN. (A) Analysis of renal function in BM-MSC–treated and HASC-treated rats after induction of anti-GBM GN. The levels of both BUN (left) and sCr (right) at day 7 after TF78 injection are significantly reduced in the HASC-treated group (_n_=7) compared with the PBS-treated (_n_=7) and BM-MSC–treated (_n_=13) groups. Dotted line indicates the value in healthy rats (_n_=3). (B) 16-hour proteinuria value on day 5 in the HASC-treated group (_n_=7) compared with the PBS-treated (_n_=7) and BM-MSC–treated (_n_=6) groups. (C and D) Representative micrographs of PAS-stained renal sections harvested on day 7. Histologic scores for glomerular crescent formation (C) and tubular injury (D) in the indicated groups (_n_=6–7 per group) at day 7 are determined semiquantitatively. Dotted areas indicate glomerular crescent formation. (E) Representative micrographs of glomeruli stained with specific monoclonal IgGs for rat CD68 (upper) and CD163 (lower) on day 7 after disease induction. Accumulation of CD68+ and CD163+ (arrowheads) macrophages per glomerulus at day 7 is also determined. All data are mean ± SD. *P<0.01 and **P<0.05 as determined by ANOVA. Scale bars indicate 50 μm, except for the micrograph illustrating tubular injury, in which the bar indicates 200 μm. PAS, periodic acid–Schiff.
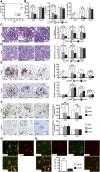
Figure 2.
Comparison of HASC and LASC administration for ASC-mediated protection against anti-GBM GN. (A) Survival curves for LASC-treated (_n_=14) and untreated (_n_=13) rats after anti-GBM antibody injection. (B) Renal function is assessed by determining BUN and sCr concentrations in PBS-treated (_n_=7), HASC-treated (_n_=7), and LASC-treated (_n_=8) anti-GBM GN rats on days 7 and 14. Dotted lines indicate value in healthy rats (_n_=3). The concentration of protein in the urine of rats in each group measured on days 3, 5, and 14 (_n_=7 per group). (C and D) Representative micrographs of kidney sections stained with PAS on day 4 to assess crescent formation (C) and on day 7 to assess tubular injury (D). Histologic scores for glomerular crescent formation and tubular injury are determined on days 4, 7, and 14 in the indicated groups (_n_=6–8 per group). Dotted areas indicate glomerular crescent formation. (E and F) Representative micrographs of glomeruli at day 4 and the interstitium at day 7, stained with specific antibodies against CD68+ and CD163+ macrophages. Glomerular (E) and interstitial (F) accumulation of CD68+ and CD163+ cells at indicated time points (_n_=7–8 per group). All data are mean ± SD. *P<0.01 and **P<0.05 as determined by ANOVA. (G) Representative micrographs of glomeruli obtained by double immunostaining for CD163 (green) and CD206 (red) at day 4 after disease induction with LASC treatment. The inset shows CD163+CD206+ macrophages under high-power magnification. (H) Representative micrographs illustrating double immunostaining for IL-10 (green) and CD163 (red) or CD206 (red) and quantification of CD163+ or CD206+ cells expressing IL-10 at day 4 after disease induction with LASC treatment. Arrowheads indicate IL10+CD163+ cells in the glomerulus and the inset shows IL-10+CD163+ cells under high-power magnification (_n_=4 per group). All data are mean ± SD. **P<0.05 as determined by t test. Scale bars, 50 μm in C and E; 100 μm in F; 200 μm in D. PAS, periodic acid–Schiff.
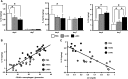
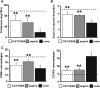
Figure 3.
Renal cytokine profiles in ASC-treated anti-GBM GN rats. (A) Concentrations of the proinflammatory cytokines IL-1_β_ and IL-12p70 and the anti-inflammatory cytokine IL-10 in the kidneys of PBS-treated, HASC-treated, and LASC-treated anti-GBM GN rats. Cytokine concentrations in nanograms per milligram of total protein in homogenates from renal cortex are determined. All data are mean ± SD. *P<0.01 as determined by ANOVA (_n_=7–8 per group). (B and C) Linear regression analysis reveals a tight correlation between renal IL-10 concentration and glomerular accumulation of CD163+ macrophages at days 4 and 7 (B) (_r_2=0.77) and sCr (C) (_r_2=0.66) at day 7. Each dot represents an individual kidney sample from a PBS-treated, HASC-treated, and LASC-treated rat.
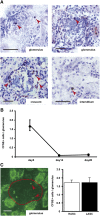
Figure 4.
Recruitment of transferred ASCs in the kidneys of anti-GBM GN rats. (A) CFSE-stained LASCs are administered to anti-GBM GN rats on days 0–4 via the tail vein, and the kidneys are harvested on day 5. Exogenous LASCs (arrowheads) in frozen sections are identified using a polyclonal anti-CFSE antibody. In kidneys, CFSE+ cells are observed in the glomeruli, glomerular crescents, and interstitium. (B) Glomerular accumulation of CFSE+ cells at days 5, 14, and 28 is shown. (C) A representative immunofluorescence image of kidney tissue from an LASC-treated anti-GBM GN rat on day 5 (left). Dotted area indicates a glomerulus. Glomerular accumulation of CFSE+ cells (arrowheads) from HASC- or LASC-treated rats is analyzed (right). No significant difference is observed between HASC-treated and LASC-treated rats with respect to the number of glomerular CFSE+ ASCs. Data are mean ± SD (_n_=4 per group). Scale bar, 50 μm.
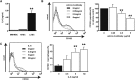
Figure 5.
ASC-mediated functional polarization of macrophages into immunoregulatory cells. (A) IL-10 concentration in culture supernatants of peritoneal macrophages cultured for 48 hours with BM-MSCs, HASCs, or LASCs at a 20:1 ratio. Dotted line indicates value in macrophages cultured alone (_n_=9–13 per group). (B) Expression of CD68 (upper) and CD163 (lower) on peritoneal macrophages cultured with MSCs at a 2:1 ratio is evaluated by flow cytometry. The percentage of CD68+ and CD163+ cells is determined. The population of CD68+ macrophages is significantly reduced and that of CD163+ macrophages is significantly increased when macrophages are cultured with LASCs compared with macrophages cultured with BM-MSCs or HASCs (_n_=7 per group). Dotted line represents value for macrophages cultured alone (_n_=7). (C) Macrophage polarization into M2 cells after direct and indirect contact with LASCs. A trans-well plate system prevents direct contact between macrophages and LASCs. Macrophages are cultured on the bottom of the plate and LASCs are cultured in the upper well at a 2:1 ratio. LASC-mediated CD163 expression on macrophages is evaluated using trans-well plates, and the percentage of CD163+ cells is determined. Macrophages with or without LASCs were subjected to positive and baseline control, respectively (_n_=7 per group). (D) Analysis of LASC-mediated polarization of macrophages into CD163+ cells under different cell ratios. The percentage of CD163+ cells is determined. (E) CD206 induction on macrophages cocultured with LASCs (left). Mean fluorescence intensity of CD206+ cells (right) is evaluated in the indicated gate. (F) Quadrants and numbers indicate percentage of cells from each gate of CD163+ and/or CD206+ macrophages alone (left) or with LASCs (right) (_n_=4 per group). All data are mean ± SD. *P<0.01 and **P<0.05 as determined by ANOVA.
Figure 6.
LASC-derived PGE2 is an essential soluble factor that promotes polarization of macrophages toward immunoregulatory cells in vitro. (A and B) The concentrations of 15d-PGJ2, a metabolite of PGD, and PGE2 in culture supernatants of MSCs cultured alone (A) or with peritoneal macrophages at a 1:2 ratio (B) are measured. Dotted line indicates the value in medium (A) and in macrophage culture supernatant (B). LASCs constitutively secrete a higher amount of PGE2 than BM-MSCs or HASCs do, and this is enhanced in coculture with macrophages (_n_=4 in each group). (C and D) The Cox-2 inhibitor CAY10404 (C) and aspirin (D) impaired PGE2 excretion by LASCs cultured with macrophages in a dose-dependent manner. Reduction of LASC-mediated macrophage polarization by CAY10404 (C) and aspirin (D) is also evaluated by flow cytometry as macrophage CD163 expression. Representative histograms and percentage of CD163+ macrophages incubated with CAY10404 or aspirin at indicated concentrations are shown. Dotted line indicates value in cultures containing macrophages alone. (E) The decrease in the CD163+ population is evaluated as the percentage of CD163+ cells cultured with each EP or PGD receptor antagonist compared with CD163+ cells cultured with the respective vehicle control (black column). (F) Macrophage polarization into CD163+ cells after a single stimulation with PGE2. CD163 expression on macrophages incubated for 48 hours with synthetic PGE2 at the indicated concentrations is determined by flow cytometry (_n_=4–5 per group). All data are mean ± SD. **P<0.05 as determined by ANOVA.
Figure 7.
Pharmaceutical ablation of PGE2 synthesis abrogated the therapeutic potency of LASCs. (A–C) LASCs pretreated with 50 μM CAY10404 or 1000 μM aspirin are transferred into anti-GBM GN rats. The 16-hour proteinuria level at day 5 (A), histologic score of glomerular crescent formation at day 7 (B), and glomerular accumulation (C) of CD68+ (left) and CD163+ (right) macrophages at day 7 are evaluated as described in the legend for Figure 2. Dotted lines indicate value in anti-GBM GN rats not subjected to LASC transfer (_n_=10). All data are mean ± SD. **P<0.05 compared with untreated LASC group as determined by ANOVA (_n_=7–10 per group).
Figure 8.
LASC-derived IL-6 is another soluble factor that promotes polarization of macrophages into CD163+ cells in vitro. (A) IL-6 concentration in the culture supernatants of BM-MSCs, HASCs, and LASCs incubated for 24 hours. (B) Neutralization of IL-6 using an anti-IL-6 antibody results in a dose-dependent decrease in the population of LASC-induced CD163+ macrophages compared with cultures treated with normal goat IgG (black column). (C) Recombinant IL-6 alone promotes the phenotypic switch of macrophages into CD163+ cells (_n_=5 per group). All data are mean ± SD. **P<0.05 as determined by ANOVA.
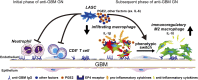
Figure 9.
Model for LASC-mediated amelioration of anti-GBM GN. LASCs impair recruitment of neutrophils and CD8+ T cells into the glomerulus during the initial phase of anti-GBM GN and promote PGE2-EP4 receptor-dependent phenotypic conversion of infiltrating macrophages to immunoregulatory macrophages during subsequent phases of the disease. Other soluble factors, including IL-6, are also required for this process. The accumulation of a significant number of immunoregulatory macrophages in the glomeruli protect against development of proteinuria and glomerular crescent formation, which is critical for a positive prognosis in anti-GBM GN.
Similar articles
- Adipose-derived stromal cells cultured in a low-serum medium, but not bone marrow-derived stromal cells, impede xenoantibody production.
Saka Y, Furuhashi K, Katsuno T, Kim H, Ozaki T, Iwasaki K, Haneda M, Sato W, Tsuboi N, Ito Y, Matsuo S, Kobayashi T, Maruyama S. Saka Y, et al. Xenotransplantation. 2011 May-Jun;18(3):196-208. doi: 10.1111/j.1399-3089.2011.00640.x. Xenotransplantation. 2011. PMID: 21696449 - Therapeutic effects of human mesenchymal stem cells in Wistar-Kyoto rats with anti-glomerular basement membrane glomerulonephritis.
Suzuki T, Iyoda M, Shibata T, Ohtaki H, Matsumoto K, Shindo-Hirai Y, Kuno Y, Wada Y, Yamamoto Y, Kawaguchi M, Shioda S, Akizawa T. Suzuki T, et al. PLoS One. 2013 Jun 24;8(6):e67475. doi: 10.1371/journal.pone.0067475. Print 2013. PLoS One. 2013. PMID: 23826305 Free PMC article. - Adipose-derived stromal/stem cells successfully attenuate the fibrosis of scleroderma mouse models.
Okamura A, Matsushita T, Komuro A, Kobayashi T, Maeda S, Hamaguchi Y, Takehara K. Okamura A, et al. Int J Rheum Dis. 2020 Feb;23(2):216-225. doi: 10.1111/1756-185X.13764. Epub 2019 Dec 5. Int J Rheum Dis. 2020. PMID: 31808305 - Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells.
Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Strioga M, et al. Stem Cells Dev. 2012 Sep 20;21(14):2724-52. doi: 10.1089/scd.2011.0722. Epub 2012 May 9. Stem Cells Dev. 2012. PMID: 22468918 Review. - Adipose Tissue-Derived Stem Cells: Immunomodulatory Effects and Therapeutic Potential.
Al-Ghadban S, Bunnell BA. Al-Ghadban S, et al. Physiology (Bethesda). 2020 Mar 1;35(2):125-133. doi: 10.1152/physiol.00021.2019. Physiology (Bethesda). 2020. PMID: 32027561 Review.
Cited by
- How Stem and Progenitor Cells Can Affect Renal Diseases.
Montenegro F, Giannuzzi F, Picerno A, Cicirelli A, Stea ED, Di Leo V, Sallustio F. Montenegro F, et al. Cells. 2024 Aug 30;13(17):1460. doi: 10.3390/cells13171460. Cells. 2024. PMID: 39273032 Free PMC article. Review. - Cell-Based Therapy for Fibrosing Interstitial Lung Diseases, Current Status, and Potential Applications of iPSC-Derived Cells.
Nakamura Y, Niho S, Shimizu Y. Nakamura Y, et al. Cells. 2024 May 22;13(11):893. doi: 10.3390/cells13110893. Cells. 2024. PMID: 38891026 Free PMC article. Review. - A novel cell source for therapy of knee osteoarthritis using atelocollagen microsphere-adhered adipose-derived stem cells: Impact of synovial fluid exposure on cell activity.
Sakamoto T, Fuku A, Horie T, Kitajima H, Nakamura Y, Tanida I, Sunami H, Hirata H, Tachi Y, Iida Y, Yamada S, Yamamoto N, Shimizu Y, Ishigaki Y, Ichiseki T, Kaneuji A, Osawa S, Kawahara N. Sakamoto T, et al. Regen Ther. 2024 Apr 23;27:408-418. doi: 10.1016/j.reth.2024.04.010. eCollection 2024 Dec. Regen Ther. 2024. PMID: 38694445 Free PMC article. - <Editors' Choice> Mesenchymal stem/stromal cells generated from induced pluripotent stem cells are highly resistant to senescence.
Aoi T, Tanaka A, Furuhashi K, Ikeya M, Shimizu A, Arioka Y, Kushima I, Ozaki N, Maruyama S. Aoi T, et al. Nagoya J Med Sci. 2023 Nov;85(4):682-690. doi: 10.18999/nagjms.85.4.682. Nagoya J Med Sci. 2023. PMID: 38155616 Free PMC article. - Synovial Fluid Derived from Human Knee Osteoarthritis Increases the Viability of Human Adipose-Derived Stem Cells through Upregulation of FOSL1.
Kitajima H, Sakamoto T, Horie T, Kuwano A, Fuku A, Taki Y, Nakamura Y, Tanida I, Sunami H, Hirata H, Tachi Y, Yamamoto N, Iida Y, Ishigaki Y, Yamada S, Shimodaira S, Shimizu Y, Ichiseki T, Kaneuji A, Osawa S, Kawahara N. Kitajima H, et al. Cells. 2023 Jan 15;12(2):330. doi: 10.3390/cells12020330. Cells. 2023. PMID: 36672268 Free PMC article.
References
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR: Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147, 1999 - PubMed
- Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA: Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 2: 83–92, 1974 - PubMed
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM: Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843, 2002 - PubMed
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R: Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 30: 42–48, 2002 - PubMed
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A: Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106: 1755–1761, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials