Evidence of small-fiber polyneuropathy in unexplained, juvenile-onset, widespread pain syndromes - PubMed (original) (raw)
Evidence of small-fiber polyneuropathy in unexplained, juvenile-onset, widespread pain syndromes
Anne Louise Oaklander et al. Pediatrics. 2013 Apr.
Abstract
Objective: We tested the hypothesis that acquired small-fiber polyneuropathy (SFPN), previously uncharacterized in children, contributes to unexplained pediatric widespread pain syndromes.
Methods: Forty-one consecutive patients evaluated for unexplained widespread pain beginning before age 21 had medical records comprehensively analyzed regarding objective diagnostic testing for SFPN (neurodiagnostic skin biopsy, nerve biopsy, and autonomic function testing), plus histories, symptoms, signs, other tests, and treatments. Healthy, demographically matched volunteers provided normal controls for SFPN tests.
Results: Age at illness onset averaged 12.3 ± 5.7 years; 73% among this poly-ethnic sample were female (P = .001). Sixty-eight percent were chronically disabled, and 68% had hospitalizations. Objective testing diagnosed definite SFPN in 59%, probable SFPN in 17%, and possible SFPN in 22%. Only 1 of 41 had entirely normal SFPN test results. Ninety-eight percent of patients had other somatic complaints consistent with SFPN dysautonomia (90% cardiovascular, 82% gastrointestinal, and 34% urologic), 83% reported chronic fatigue, and 63% had chronic headache. Neurologic examinations identified reduced sensation in 68% and vasomotor abnormalities in 55%, including 23% with erythromelalgia. Exhaustive investigations for SFPN causality identified only history of autoimmune illnesses in 33% and serologic markers of disordered immunity in 89%. Treatment with corticosteroids and/or intravenous immune globulin objectively and subjectively benefited 80% of patients (12/15).
Conclusions: More than half among a large series of patients with childhood-onset, unexplained chronic widespread pain met rigorous, multitest, diagnostic criteria for SFPN, which extends the age range of acquired SFPN into early childhood. Some cases appeared immune-mediated and improved with immunomodulatory therapies.
Figures
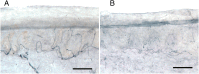
FIGURE 1
Loss of PGP 9.5 immunolabeled nerve fibers in vertical sections from distal leg skin biopsy. A, Biopsy from a 19-year-old white male healthy control contains abundant innervation (675 ENF/mm2 skin surface area). B, Biopsy from a 19-year-old white male patient demonstrates reduced epidermal (155 ENF/mm2) and dermal nerve fibers. SFPN was confirmed by skin biopsy; ENF density = 1.3th percentile of laboratory norms. Bars represent 50 μm.
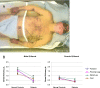
FIGURE 2
A, Patient with delayed sweating on torso and arms during thermoregulatory sweat testing. This 20 year old had non–length-dependent erythromelalgia (pain worsened by heat, plus redness and swelling) that was worse in his cheeks and ears but also affected his hands and feet, labile heart rate and blood pressure, and diarrhea. His distal leg skin biopsy had minor abnormalities, and antinuclear antibodies were present at a titer of 1:80. Thermoregulatory sweat testing involves applying Alizarin red indicator to the skin before controlled heating; it is orange when dry and turns purple when wet. When performed at the Mayo Clinic this revealed delayed sweating on the torso and arms compared with the hands and thighs. This patient’s AFT results were normal at the Mayo Clinic but abnormal at Cleveland Clinic and at the National Institutes of Health, with reduced sweating at forearm and distal leg study sites. His patchy/proximal symptoms and test results were consistent with non–length-dependent neuronopathy/ganglionopathy. Image courtesy of Paola Sandroni, MD, PhD, Mayo Clinic. B, Gender- and site-specific comparison of acetylcholine-evoked sweat production in normal controls and patients. All values from patients (n = 33, 10 boys and 23 girls) were compared with all values from gender- and age range–matched controls (n = 38; 19 boys and 19 girls). Symbols depict mean sweat volumes at each study site ± SEM; lines connect same-site groups of controls and patients. Sweating was reduced in male patients versus male controls at the forearm (P = .0011) and proximal leg (P = .0017). Sweating was reduced in female patients versus female controls at the forearm (P = .024), proximal leg (P = .024), and foot (P = .009).
FIGURE 3
This 19 year old had widespread pain, tachycardia, orthostatic hypotension, headache, nausea, and obstipation since childhood. Blood tests identified type III cryoglobulinemia (IgG, IgM, C3), no hepatitis, low complement (C4 = 6, 7, 11; RR, 16–38 mg/dL; C3 = 35, 52; RR, 86–184 mg/dL; ESR = 20–26; RR, 0–17 mm/hour). SFPN was confirmed by skin biopsy; ENF density = 2.4th centile (not shown). Photomicrographs courtesy of Cynthia Magro, MD, Weill Cornell Medical College (vertical sections, 4 µm; 40× magnification). A, Hematoxylin and eosin staining depicting endothelial cell swelling and basement membrane thickening indicating chronic insidious microvasculopathy. B, Immunohistochemical reactivity of dermal microvessels for C4d, a stable component of classic complement activation and a likely correlate of her low serum C4. C, Direct immunofluorescent labeling of IgM deposits on dermal microvessels, a nonspecific marker of microvasculopathy consistent with deposition of the IgM immune complexes present in her blood.
Similar articles
- Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia.
Oaklander AL, Herzog ZD, Downs HM, Klein MM. Oaklander AL, et al. Pain. 2013 Nov;154(11):2310-2316. doi: 10.1016/j.pain.2013.06.001. Epub 2013 Jun 5. Pain. 2013. PMID: 23748113 Free PMC article. - Validation of the composite autonomic symptom scale 31 (COMPASS-31) in patients with and without small fiber polyneuropathy.
Treister R, O'Neil K, Downs HM, Oaklander AL. Treister R, et al. Eur J Neurol. 2015 Jul;22(7):1124-30. doi: 10.1111/ene.12717. Epub 2015 Apr 23. Eur J Neurol. 2015. PMID: 25907824 Free PMC article. - Small Fiber Polyneuropathy Is Prevalent in Patients Experiencing Complex Chronic Pelvic Pain.
Chen A, De E, Argoff C. Chen A, et al. Pain Med. 2019 Mar 1;20(3):521-527. doi: 10.1093/pm/pny001. Pain Med. 2019. PMID: 29447372 - Immunotherapy Prospects for Painful Small-fiber Sensory Neuropathies and Ganglionopathies.
Oaklander AL. Oaklander AL. Neurotherapeutics. 2016 Jan;13(1):108-17. doi: 10.1007/s13311-015-0395-1. Neurotherapeutics. 2016. PMID: 26526686 Free PMC article. Review. - [SMALL FIBER POLYNEUROPATHY IN YOUNG PATIENTS].
Treister R, Mirski Y, Honigman L, Yarnitsky D. Treister R, et al. Harefuah. 2020 Mar;159(3):181-185. Harefuah. 2020. PMID: 32186788 Review. Hebrew.
Cited by
- A Review of the Therapeutic Targeting of SCN9A and Nav1.7 for Pain Relief in Current Human Clinical Trials.
Dormer A, Narayanan M, Schentag J, Achinko D, Norman E, Kerrigan J, Jay G, Heydorn W. Dormer A, et al. J Pain Res. 2023 May 4;16:1487-1498. doi: 10.2147/JPR.S388896. eCollection 2023. J Pain Res. 2023. PMID: 37168847 Free PMC article. Review. - Association of small-fiber polyneuropathy with three previously unassociated rare missense SCN9A variants.
Kelley MA, Oaklander AL. Kelley MA, et al. Can J Pain. 2020;4(1):19-29. doi: 10.1080/24740527.2020.1712652. Epub 2020 Feb 5. Can J Pain. 2020. PMID: 32719824 Free PMC article. - Implications of Nerve Fiber Density on the Diagnosis and Treatment of Juvenile Fibromyalgia.
Ahmed N, Vigouroux M, Ingelmo P. Ahmed N, et al. J Pain Res. 2022 Feb 17;15:513-520. doi: 10.2147/JPR.S340038. eCollection 2022. J Pain Res. 2022. PMID: 35210850 Free PMC article. Review. - Lengthened Cutaneous Silent Period in Fibromyalgia Suggesting Central Sensitization as a Pathogenesis.
Baek SH, Seok HY, Koo YS, Kim BJ. Baek SH, et al. PLoS One. 2016 Feb 12;11(2):e0149248. doi: 10.1371/journal.pone.0149248. eCollection 2016. PLoS One. 2016. PMID: 26871583 Free PMC article. - Antiplexin D1 Antibodies Relate to Small Fiber Neuropathy and Induce Neuropathic Pain in Animals.
Fujii T, Lee EJ, Miyachi Y, Yamasaki R, Lim YM, Iinuma K, Sakoda A, Kim KK, Kira JI. Fujii T, et al. Neurol Neuroimmunol Neuroinflamm. 2021 Jun 7;8(5):e1028. doi: 10.1212/NXI.0000000000001028. Print 2021 Jul. Neurol Neuroimmunol Neuroinflamm. 2021. PMID: 34099459 Free PMC article.
References
- Wolfe F, Smythe HA, Yunus MB, et al. Report of the Multicenter Criteria Committee . The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum. 1990;33(2):160–172 - PubMed
- Buskila D. Pediatric fibromyalgia. Rheum Dis Clin North Am. 2009;35(2):253–261 - PubMed
- van Geelen SM, Bakker RJ, Kuis W, van de Putte EM. Adolescent chronic fatigue syndrome: a follow-up study. Arch Pediatr Adolesc Med. 2010;164(9):810–814 - PubMed
- Mikkelsson M, El-Metwally A, Kautiainen H, Auvinen A, Macfarlane GJ, Salminen JJ. Onset, prognosis and risk factors for widespread pain in schoolchildren: a prospective 4-year follow-up study. Pain. 2008;138(3):681–687 - PubMed
- Ojha A, Chelimsky TC, Chelimsky G. Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr. 2011;158(1):20–23 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous