Using macrophage activation to augment immunotherapy of established tumours - PubMed (original) (raw)
Using macrophage activation to augment immunotherapy of established tumours
Z G Fridlender et al. Br J Cancer. 2013.
Abstract
Background: Successful immunotherapy will require alteration of the tumour microenvironment and/or decreased immune suppression. Tumour-associated macrophages (TAMs) are one major factor affecting tumour microenvironment. We hypothesised that altering TAM phenotype would augment the efficacy of immunotherapy.
Methods: We and others have reported that 5,6-Dimethylxanthenone-4-acetic-acid (DMXAA, Vadimezan) has the ability to change TAM phenotypes, inducing a tumour microenvironment conducive to antitumour immune responses. We therefore combined DMXAA with active immunotherapies, and evaluated anti-tumour efficacy, immune cell phenotypes (flow cytometry), and tumour microenvironment (RT-PCR).
Results: In several different murine models of immunotherapy for lung cancer, DMXAA-induced macrophage activation significantly augmented the therapeutic effects of immunotherapy. By increasing influx of neutrophils and anti-tumour (M1) macrophages to the tumour, DMXAA altered myeloid cell phenotypes, thus changing the intratumoural M2/non-M2 TAM immunoinhibitory ratio. It also altered the tumour microenvironment to be more pro-inflammatory. Modulating macrophages during immunotherapy resulted in increased numbers, activity, and antigen-specificity of intratumoural CD8(+) T cells. Macrophage depletion reduced the effect of combining immunotherapy with macrophage activation, supporting the importance of TAMs in the combined effect.
Conclusion: Modulating intratumoural macrophages dramatically augmented the effect of immunotherapy. Our observations suggest that addition of agents that activate TAMs to immunotherapy should be considered in future trials.
Figures
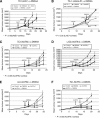
Figure 1
5,6-Dimethylxanthenone-4-acetic-acid (DMXAA) significantly augments tumour immunotherapy. Mice bearing large flank tumours were treated in one of the four ways: (1) no treatment (control), (2) i.p. DMXAA (single dose), (3) immunotherapy, and (4) a combination of immunotherapy and DMXAA (Combo). In all models presented, combination therapies led to clear tumour regression compared with each treatment alone. The following combinations of immunotherapy and DMXAA in murine lung cancer cell lines are shown: (A) the TC1 cell line with adenovirus expressing HPV-E7 (Ad.E7). (B) The TC1 cell line with a modified listeria vector expressing HPV-E7 (Lm-E7). (C) The TC1 cell line with adenovirus expressing interferon-β (Ad.IFN_β_). (D) The L1C2 cell line with adenovirus expressing interferon-β (Ad.IFN_β_). (E) The LLC cell line with adenovirus expressing interferon-β (Ad.IFN_β_). (F) The TC1 cell line with adenovirus expressing interferon-γ (Ad.IFN_γ_). *P<0.05 in combination therapy versus immunotherapy alone.
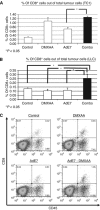
Figure 2
5,6-Dimethylxanthenone-4-acetic-acid (DMXAA) alters the myeloid cell phenotypes induced by immunotherapy. Mice (n_=4–6 for each group) bearing large TC-1 or LLC tumours were treated in one of the four ways: (1) no treatment (Control), (2) i.p. DMXAA, (3) s.q. vaccine with Ad.E7 in the TC1 cell line (Ad.E7) or Ad.IFN_β in the LLC cell line, and (4) combination of Ad.E7/Ad.IFN_β_ and DMXAA. At 3 days after the injection of DMXAA, tumours were harvested. (A) The percentage of different myeloid cells out of all tumour cells in the TC1 cell line model is summarised. Combo therapy, DMXAA, and Ad.E7 all significantly (P<0.05) increased macrophages (defined as CD11b+/CD11c-/Ly6G−) and DCs (defined as CD11b+/CD11c+/Ly6G−) compared with control tumours to a similar extent. Neutrophils (defined as CD11b+/CD11c−/Ly6G+) were increased in the combination group (*P<0.05) compared with control or either treatment alone. (B) The percentage of classically and alternatively activated macrophages (defined as CD11b+/F480+ and CD206− or CD206+, respectively) is summarised. The DMXAA significantly increased the percentage of the classically activated ‘M1' macrophages by approximately two-fold compared with vaccine alone (&P<0.05 between Combo and either control or Ad.E7) without changing the alternatively activated ‘M2' macrophages. The left panel shows TC1/Ad.E7 model and the right panel shows LLC/Ad.IFN_β_ model. (C) Representative FACS tracings of F4/80 versus CD206 in macrophages in each group in the TC1/Ad.E7 model are shown. The numbers in each quadrant are the percentages of M1 (F4/80+/CD206−) and M2 (F4/80+/CD206+) macrophages. (D) The calculated ‘immuno-stimulation ratio', that is, the ratio of non-M2 TAMs (antitumour) to M2 TAMs (protumour), is shown. The top panel shows TC1/Ad.E7 model and the bottom panel shows LLC/Ad.IFN_β_ model. Following combined treatment, this ratio increased markedly (*P<0.05 compared with all other treatments in both models).
Figure 3
5,6-Dimethylxanthenone-4-acetic-acid (DMXAA) in mice treated with immunotherapy increases the number of intratumoural CD8+ T cells. Mice (n_=4–6 for each group) bearing large TC-1 or LLC tumours were treated as in Figure 2. At 3 days after the injection of DMXAA, tumours were harvested. (A and B) The percentage of intratumoural CD8+ cells of total tumour by flow cytometry in the TC1/Ad.E7 model (A) and the LLC/Ad.IFN_β model (B). In both models, DMXAA combined with the vaccine more than doubled the percentage of intratumoural CD8+ cells compared with vaccine alone (*P<0.05). (C) Representative FACS tracings of CD45+ versus CD8+ cells in the TC1/Ad.E7 model are shown. The number in each upper-right quadrant is the percentage of CD8+ cells out of all tumour cells.
Figure 4
5,6-Dimethylxanthenone-4-acetic-acid (DMXAA) in mice treated with immunotherapy increases the activity and specificity of intratumoural CD8+ T cells. Mice (_n_=4–6 for each group) bearing large TC-1 or LLC tumours were treated as in Figure 2. At 3 days after the injection of DMXAA, tumours were harvested. (A and B) The percentage of activated intratumoural CD8+ T cells defined by expression of the activation markers 41BB (A) and CD25 (B). In the TC1/Ad.E7 model (A, left and B, left), CD8+ cell activation was increased in all treatment groups compared with control (*P<0.05). The percentage of activated CD8+ cells was increased in the combination treatment compared with Ad.E7 alone (&P_=0.06). In the LLC/Ad.IFN_β model (A, right and B, right), CD8+ cell activation was increased in the combination group compared with all other treatments (*P<0.05). (C) The percentage of intratumoural E7-specific CD8+ cells (Tet+). Combining DMXAA with Ad.E7 vaccine increased the percentage of tetramer-positive CD8+ cells by four-fold compared with control (**P<0.01) and two-fold compared with vaccine alone (*P<0.05) and DMXAA alone (_P_=0.057).
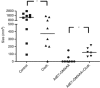
Figure 5
Depletion of macrophages reduces the effect of combining immunotherapy with DMXAA. Mice bearing large TC1 flank tumours were treated in one of the four ways: (1) ‘empty' liposomes (control), (2) i.p. and intratumoural (i.t.) Clodronate liposomes (Clodr.), (3) combination of Ad.E7 vaccine+DMXAA with empty liposomes (Ad.E7+DMXAA), and (4) combination of Ad.E7 vaccine+DMXAA with Clodronate liposomes (Ad.E7+DMXAA+Clodr.). Treatment with Clodronate liposomes reduced tumour size compared with mice treated with unloaded liposomes (*_P_=0.05). As previously shown, treatment with the combination of Ad.E7 and DMXAA markedly reduced tumour size, with 6 out of 8 cured. Addition of Clodronate liposomes inhibited the therapeutic efficacy of combination therapy (*P<0.05). Each dot represents one mouse.
Similar articles
- Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma.
Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR, Albelda SM. Jassar AS, et al. Cancer Res. 2005 Dec 15;65(24):11752-61. doi: 10.1158/0008-5472.CAN-05-1658. Cancer Res. 2005. PMID: 16357188 - 5,6-Dimethylxanthenone-4-acetic acid treatment of a non-immunogenic tumour does not synergize with active or passive CD8+ T-cell immunotherapy.
Matthews KE, Hermans IF, Roberts JM, Ching LM, Ronchese F. Matthews KE, et al. Immunol Cell Biol. 2006 Aug;84(4):383-9. doi: 10.1111/j.1440-1711.2006.01448.x. Immunol Cell Biol. 2006. PMID: 16834573 - Macrophage targeting: opening new possibilities for cancer immunotherapy.
Cassetta L, Kitamura T. Cassetta L, et al. Immunology. 2018 Nov;155(3):285-293. doi: 10.1111/imm.12976. Epub 2018 Jul 31. Immunology. 2018. PMID: 29963704 Free PMC article. Review. - Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy.
Toledo B, Zhu Chen L, Paniagua-Sancho M, Marchal JA, Perán M, Giovannetti E. Toledo B, et al. J Hematol Oncol. 2024 Jun 11;17(1):44. doi: 10.1186/s13045-024-01559-0. J Hematol Oncol. 2024. PMID: 38863020 Free PMC article. Review.
Cited by
- Combination of anti-vascular agent - DMXAA and HIF-1α inhibitor - digoxin inhibits the growth of melanoma tumors.
Smolarczyk R, Cichoń T, Pilny E, Jarosz-Biej M, Poczkaj A, Kułach N, Szala S. Smolarczyk R, et al. Sci Rep. 2018 May 9;8(1):7355. doi: 10.1038/s41598-018-25688-y. Sci Rep. 2018. PMID: 29743548 Free PMC article. - FGL2 as a Multimodality Regulator of Tumor-Mediated Immune Suppression and Therapeutic Target in Gliomas.
Yan J, Kong LY, Hu J, Gabrusiewicz K, Dibra D, Xia X, Heimberger AB, Li S. Yan J, et al. J Natl Cancer Inst. 2015 May 13;107(8):djv137. doi: 10.1093/jnci/djv137. Print 2015 Aug. J Natl Cancer Inst. 2015. PMID: 25971300 Free PMC article. - Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer.
Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, Conejo-Garcia JR, Feldman M, Albelda SM, Singhal S. Eruslanov EB, et al. J Clin Invest. 2014 Dec;124(12):5466-80. doi: 10.1172/JCI77053. Epub 2014 Nov 10. J Clin Invest. 2014. PMID: 25384214 Free PMC article. Clinical Trial. - Along the Axis between Type 1 and Type 2 Immunity; Principles Conserved in Evolution from Fish to Mammals.
Yamaguchi T, Takizawa F, Fischer U, Dijkstra JM. Yamaguchi T, et al. Biology (Basel). 2015 Nov 17;4(4):814-59. doi: 10.3390/biology4040814. Biology (Basel). 2015. PMID: 26593954 Free PMC article. Review. - Control of immune cell entry through the tumour vasculature: a missing link in optimising melanoma immunotherapy?
Tan LY, Martini C, Fridlender ZG, Bonder CS, Brown MP, Ebert LM. Tan LY, et al. Clin Transl Immunology. 2017 Mar 17;6(3):e134. doi: 10.1038/cti.2017.7. eCollection 2017 Mar. Clin Transl Immunology. 2017. PMID: 28435677 Free PMC article. Review.
References
- Aharinejad S, Paulus P, Sioud M, Hofmann M, Zins K, Schafer R, Stanley ER, Abraham D. Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res. 2004;64:5378–5384. - PubMed
- Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. - PubMed
- Ching LM, Young HA, Eberly K, Yu CR. Induction of STAT and NFkappaB activation by the antitumor agents 5,6-dimethylxanthenone-4-acetic acid and flavone acetic acid in a murine macrophage cell line. Biochem Pharmacol. 1999;58:1173–1181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA066726/CA/NCI NIH HHS/United States
- P01 CA 66726/CA/NCI NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- ES013508-02/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials