A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation - PubMed (original) (raw)
A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation
Hsi-Min Hsiao et al. PLoS One. 2013.
Abstract
Introduction: Cigarette smoke is a profound pro-inflammatory stimulus that contributes to acute lung injuries and to chronic lung disease including COPD (emphysema and chronic bronchitis). Until recently, it was assumed that resolution of inflammation was a passive process that occurred once the inflammatory stimulus was removed. It is now recognized that resolution of inflammation is a bioactive process, mediated by specialized lipid mediators, and that normal homeostasis is maintained by a balance between pro-inflammatory and pro-resolving pathways. These novel small lipid mediators, including the resolvins, protectins and maresins, are bioactive products mainly derived from dietary omega-3 and omega-6 polyunsaturated fatty acids (PUFA). We hypothesize that resolvin D1 (RvD1) has potent anti-inflammatory and pro-resolving effects in a model of cigarette smoke-induced lung inflammation.
Methods: Primary human lung fibroblasts, small airway epithelial cells and blood monocytes were treated with IL-1β or cigarette smoke extract in combination with RvD1 in vitro, production of pro-inflammatory mediators was measured. Mice were exposed to dilute mainstream cigarette smoke and treated with RvD1 either concurrently with smoke or after smoking cessation. The effects on lung inflammation and lung macrophage populations were assessed.
Results: RvD1 suppressed production of pro-inflammatory mediators by primary human cells in a dose-dependent manner. Treatment of mice with RvD1 concurrently with cigarette smoke exposure significantly reduced neutrophilic lung inflammation and production of pro-inflammatory cytokines, while upregulating the anti-inflammatory cytokine IL-10. RvD1 promoted differentiation of alternatively activated (M2) macrophages and neutrophil efferocytosis. RvD1 also accelerated the resolution of lung inflammation when given after the final smoke exposure.
Conclusions: RvD1 has potent anti-inflammatory and pro-resolving effects in cells and mice exposed to cigarette smoke. Resolvins have strong potential as a novel therapeutic approach to resolve lung injury caused by smoke and pulmonary toxicants.
Conflict of interest statement
Competing Interests: TT is a PLOS One Editorial Board Member. CS is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. CS is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. CNS’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials. No other authors have competing interests to declare.
Figures
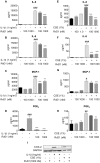
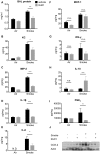
Figure 1. RvD1 inhibits IL-1β-induced and CSE-induced cytokine and chemokine production from primary human lung fibroblasts.
Primary human lung fibroblasts were pre-treated with the indicated concentrations of RvD1 for 24 hours then stimulated with IL-1β (1 ng/ml) or cigarette smoke extract (CSE, 1%) for an additional 24 hours. Culture media from IL-1β-treated cells (A–D) or CSE-treated cells (E–H) were subjected to ELISA and EIA to detect various pro-inflammatory mediators as described in the Methods. Data shown are mean ± SD for n = 4 replicate cultures, from one representative experiment of 3 performed. COX-2 and GAPDH expression (I) were determined in cell lysates by Western blotting. Blots shown are representative of three independent experiments, and the lanes were run non-contiguously on the same gel. ***P<0.001, **p<0.01 *P<0.05 compared to IL-1β-treated or CSE-treated cultures without RvD1, ###P<0.001 versus vehicle-treated control cultures by one-way ANOVA with Bonferroni post-tests.
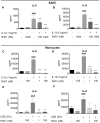
Figure 2. RvD1 inhibits IL-1β and CSE-induced cytokine production from primary human small airway epithelial cells and monocytes.
Primary SAEC (A–B) were pre-treated with indicated concentrations of RvD1 for 24 hours then stimulated with IL-1β (1 ng/ml) for an additional 24 hours. Culture media was collected and levels of IL-6 (A) and IL-8 (B) were determined by ELISA. Data shown are mean ± SD of triplicate cultures, from one representative experiment of 3 performed. Blood-derived monocytes (C–F) were pre-treated with indicated concentrations of RvD1 for 24 hours then stimulated with IL-1β (1 ng/ml) or CSE (5%) for an additional 24 hours. Culture media was collected and levels of IL-6 (C, E) and IL-8 (D, F) were determined by ELISA. Data shown are mean ± SEM for three individual donors. ##p<0.01, ###p<0.001 compared to vehicle-treated control cultures. **p<0.01, ***p<0.001 compared to IL-1β or CSE without RvD1, using one-way ANOVA with Bonferroni post-tests.
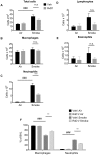
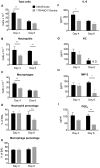
Figure 3. RvD1 attenuates CS-induced inflammation in vivo.
Mice were treated with 100 ng RvD1 (grey bars) or saline vehicle (Veh, black bars) by inhalation 1 hour prior to cigarette smoke, daily for 3 days. Mice were euthanized at day 4, and differential cell counts were performed on BALF cells. Total cell number (A), number of macrophages (B), neutrophils (C), lymphocytes (D), eosinophils (E) and percentage of macrophages and neutrophils (F) are reported. Data are shown as mean ± SEM for n = 6–7 mice per group from one of 3 independent experiments. ***P<0.001, versus smoke-exposed mice by two-way ANOVA with Bonferroni post-tests. ###P<0.001, versus air-exposed control mice by one-way ANOVA with Bonferroni post-tests.
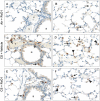
Figure 4. RvD1 reduces CS-induced neutrophilic lung inflammation.
Lungs from mice that had not been lavaged were inflated and fixed with neutral buffered formalin, and 5 µm paraffin sections were stained with a neutrophil-specific antibody (brown) and counter-stained with hematoxylin. An occasional neutrophil was observed in air-exposed mice treated with RvD1 (A, B), similar to air-exposed mice treated with vehicle (not shown). CS induces a neutrophilic inflammation around vessels and airways (C) and in the parenchyma (D), with neutrophils prominent in the interstitial spaces (arrows) and the alveoli (solid triangles). RvD1 treatment reduces perivascular and peribronchial (E) as well as parenchymal (F) accumulation of neutrophils. A, airway; v, vessel. Arrows, interstitial neutrophils; solid triangles, neutrophils in airspaces.
Figure 5. RvD1 decreases production of pro-inflammatory mediators in BALF and total lung homogenate but increases IL-10.
Total protein (A) in BALF was measured by BCA assay. Neutrophil chemoattractants KC (B) and MIP-2 (C), IL-1β (D), IL-6 (E), MCP-1 (F), IFN-γ (G), PGE2 (H) and IL-10 (I) were determined in lung homogenates by multiplex assay or EIA and normalized to total protein concentration. N.D., not detected. Data are shown as mean ± SEM for n = 6–8 mice per group and are representative of 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001 versus smoke-exposed mice by two-way ANOVA with Bonferroni post-tests. ###P<0.001 compared to air-exposed control mice by one-way ANOVA with Bonferroni post-tests. Expression of COX-2 and total actin (J) were determined in whole lung homogenates by Western blotting using COX-2 and total actin specific antibodies. Each lane represents an individual mouse.
Figure 6. 17R-RvD1 promotes the resolution of acute lung inflammation induced by CS exposure.
Mice were exposed to CS exposure on days 1, 2 and 3. The mice received 100 ng 17R-RvD1 (grey bars) or saline vehicle (Veh, black bars) by inhalation on day 3 (1 hour after the last cigarette smoke exposure), day 4 and day 5. Mice were euthanized at days 4 and 6 and differential cell counts were performed on BAL cells. Total cell number (A), number of neutrophils (B), and macrophages (C), percentage of neutrophils (D) and macrophages (E) are reported. BALF was collected and analyzed by ELISA or EIA for IL-6 (F), KC (G), MIP-2 (H) and PGE2 (I). Data are shown as mean ± SEM for n = 4–5 mice per group. N.D., not detected. *P<0.05, **P<0.01 compared to Vehicle/Smoke mice by two-way ANOVA with Bonferroni post-tests.
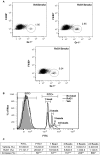
Figure 7. RvD1 enhances macrophage phagocytosis in vivo and ex vivo.
Mice were exposed to air or CS with or without RvD1 as described. Lungs were digested and macrophages were isolated as described in Methods. The percentage of F4/80/Gr-1 double positive cells was quantified by flow cytometry. (A) Dot plots shown are from one vehicle control mouse and two RvD1-treated mice. (B) BAL cells were harvested from naïve mice and incubated ex vivo with 1 ng/ml IL-1β and 100 nM RvD1 for 30 minutes prior to the addition of FITC-labeled latex beads. Percentage of F4/80+ macrophages that ingested FITC-latex beads was quantified by flow cytometry. Representative histogram of one out of 6 mice is shown. (C) Mean ± SEM is shown for 6 animals from two independent experiments. *p<0.05, **p<0.01, ***p<0.001, Student’s t-test.
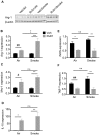
Figure 8. RvD1 drives polarization of alternatively activated macrophages.
Mice were exposed to air or CS with or without RvD1 as described. (A) Expression of Arg-1 was measured in whole lung homogenates by Western blotting. Each lane represents one animal. B–F: Alveolar macrophages were enriched from BAL cells by adherence and total RNA was extracted and subjected to quantitative RT-PCR to measure mRNA levels of Arg1 (B), Mrc1 (C), IL-10 (D), iNOS (E) and TNFα (F). Mean ± SEM is shown, normalized to 18S rRNA. Each group n = 6 for Arg1, Mrc1 and IL-10; n = 3 for iNOS and TNFα. #P<0.05, ##P<0.01, compared to Vehicle/Air animal by one-way ANOVA with Bonferroni post-tests; *P<0.05, **P<0.01, ***P<0.001 versus Vehicle/Smoke animal by two-way ANOVA with Bonferroni post-test. ‡P<0.05 between RvD1/Air and RvD1/Smoke, ANOVA.
Similar articles
- Resolvin D1 prevents smoking-induced emphysema and promotes lung tissue regeneration.
Kim KH, Park TS, Kim YS, Lee JS, Oh YM, Lee SD, Lee SW. Kim KH, et al. Int J Chron Obstruct Pulmon Dis. 2016 May 27;11:1119-28. doi: 10.2147/COPD.S100198. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27313451 Free PMC article. - Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages.
Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, Phipps RP. Croasdell A, et al. Am J Physiol Lung Cell Mol Physiol. 2015 Oct 15;309(8):L888-901. doi: 10.1152/ajplung.00125.2015. Epub 2015 Aug 21. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26301452 Free PMC article. - AT-RvD1 Mitigates Secondhand Smoke-Exacerbated Pulmonary Inflammation and Restores Secondhand Smoke-Suppressed Antibacterial Immunity.
Bhat TA, Kalathil SG, Bogner PN, Lehmann PV, Thatcher TH, Sime PJ, Thanavala Y. Bhat TA, et al. J Immunol. 2021 Mar 15;206(6):1348-1360. doi: 10.4049/jimmunol.2001228. Epub 2021 Feb 8. J Immunol. 2021. PMID: 33558371 Free PMC article. - Resolvin D1: A key endogenous inhibitor of neuroinflammation.
Roohbakhsh A, Etemad L, Karimi G. Roohbakhsh A, et al. Biofactors. 2022 Sep;48(5):1005-1026. doi: 10.1002/biof.1891. Epub 2022 Sep 29. Biofactors. 2022. PMID: 36176016 Review. - Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome.
Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Serhan CN, et al. Biochim Biophys Acta. 2015 Apr;1851(4):397-413. doi: 10.1016/j.bbalip.2014.08.006. Epub 2014 Aug 17. Biochim Biophys Acta. 2015. PMID: 25139562 Free PMC article. Review.
Cited by
- Aspirin-Triggered Resolvin D1 Versus Dexamethasone in the Treatment of Sjögren's Syndrome-Like NOD/ShiLtJ Mice - A Pilot Study.
Easley JT, Nelson JW, Mellas RE, Sommakia S, Wu C, Trump B, Baker OJ. Easley JT, et al. J Rheum Dis Treat. 2015;1(4):027. doi: 10.23937/2469-5726/1510027. Epub 2015 Dec 17. J Rheum Dis Treat. 2015. PMID: 27110599 Free PMC article. - Enhanced silver nanoparticle-induced pulmonary inflammation in a metabolic syndrome mouse model and resolvin D1 treatment.
Alqahtani S, Xia L, Shannahan JH. Alqahtani S, et al. Part Fibre Toxicol. 2022 Aug 6;19(1):54. doi: 10.1186/s12989-022-00495-6. Part Fibre Toxicol. 2022. PMID: 35933425 Free PMC article. - Resolution of inflammation in periodontitis: a review.
Huang J, Cai X, Ou Y, Zhou Y, Wang Y. Huang J, et al. Int J Clin Exp Pathol. 2018 Sep 1;11(9):4283-4295. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31949825 Free PMC article. Review. - Comparison of in vitro toxicological effects of biomass smoke from different sources of animal dung.
McCarthy CE, Duffney PF, Wyatt JD, Thatcher TH, Phipps RP, Sime PJ. McCarthy CE, et al. Toxicol In Vitro. 2017 Sep;43:76-86. doi: 10.1016/j.tiv.2017.05.021. Epub 2017 May 30. Toxicol In Vitro. 2017. PMID: 28572013 Free PMC article. - Are specialized pro-resolving mediators promising therapeutic agents for severe bronchial asthma?
Hisada T, Aoki-Saito H, Koga Y. Hisada T, et al. J Thorac Dis. 2017 Nov;9(11):4266-4269. doi: 10.21037/jtd.2017.10.116. J Thorac Dis. 2017. PMID: 29268487 Free PMC article. No abstract available.
References
- Cosio MG, Saetta M, Agusti A (2009) Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 360: 2445–2454. - PubMed
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, et al. (2007) Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. The Journal of biological chemistry 282: 9323–9334. - PubMed
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, et al. (2005) Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proceedings of the National Academy of Sciences of the United States of America 102: 7671–7676. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL088325/HL/NHLBI NIH HHS/United States
- ES01247/ES/NIEHS NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- ES07026/ES/NIEHS NIH HHS/United States
- R01 ES007026/ES/NIEHS NIH HHS/United States
- HL088325-02S1/HL/NHLBI NIH HHS/United States
- UL1 RR024160/RR/NCRR NIH HHS/United States
- 8UL1TR000042/TR/NCATS NIH HHS/United States
- T32 ES007026/ES/NIEHS NIH HHS/United States
- HL66988/HL/NHLBI NIH HHS/United States
- T32 HL066988/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical