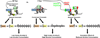Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism - PubMed (original) (raw)
Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism
Sachin Moonat et al. Biol Psychiatry. 2013.
Abstract
Background: Epigenetic mechanisms have been implicated in psychiatric disorders, including alcohol dependence. However, the epigenetic basis and role of specific histone deacetylase (HDAC) isoforms in the genetic predisposition to anxiety and alcoholism is unknown.
Methods: We measured amygdaloid HDAC activity, levels of HDAC isoforms, and histone H3 acetylation in selectively bred alcohol-preferring (P) and -nonpreferring (NP) rats. We employed HDAC2 small interfering RNA infusion into the central nucleus of amygdala (CeA) of P rats to determine the causal role of HDAC2 in anxiety-like and alcohol-drinking behaviors. Chromatin immunoprecipitation analysis was performed to examine the histone acetylation status of brain-derived neurotrophic factor (Bdnf) and activity-regulated cytoskeleton associated protein (Arc) genes. Golgi-Cox staining was performed to measure dendritic spine density.
Results: We found that P rats innately display higher nuclear HDAC activity and HDAC2 but not HDAC 1, 3, 4, 5, and 6 protein levels and lower acetylation of H3-K9 but not H3-K14, in the CeA and medial nucleus of amygdala compared with NP rats. Acute ethanol exposure decreased amygdaloid HDAC activity and HDAC2 protein levels, increased global and gene (Bdnf and Arc)-specific histone acetylation, and attenuated anxiety-like behaviors in P rats but had no effects in NP rats. The HDAC2 knockdown in the CeA attenuated anxiety-like behaviors and voluntary alcohol but not sucrose consumption in P rats and increased histone acetylation of Bdnf and Arc with a resultant increase in protein levels that correlated with increased dendritic spine density.
Conclusions: These novel data demonstrate the role of HDAC2-mediated epigenetic mechanisms in anxiety and alcoholism.
Published by Elsevier Inc.
Figures
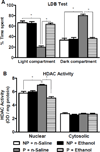
Figure 1. Effects of acute ethanol exposure on anxiety-like behaviors and HDAC activity in the amygdala of P and NP rats
A. The light/dark box (LDB) exploration test showed that alcohol-preferring (P) rats display innate anxiety-like behaviors in comparison to alcohol-nonpreferring (NP) rats. Acute ethanol treatment (1 g/kg) produced anxiolytic effects in P rats, but not NP rats. Values are represented as the mean ± SEM of the percentage of time spent in each compartment averaged from 13 rats per group. *Significantly different from respective control groups (Two-way ANOVA; group × treatment, F1,48 = 58.6, p<0.001 followed by post hoc analysis by Tukey’s test, p<0.001). B. The nuclear fraction, but not cytosolic fraction, of the amygdala from P rats displays higher histone deacetylase (HDAC) activity at baseline compared to NP rats. Acute ethanol treatment inhibited the nuclear HDAC activity in the amygdala of P rats, without any effect in NP rats. Values are represented as the mean ± SEM of the optical density (OD) per mg of protein from 6 rats per group. *Significantly different from respective control groups (Nuclear HDAC activity, two-way ANOVA; group × treatment, F1, 20 = 29.6, p<0.001 followed by post hoc analysis by Tukey’s test, p<0.001).
Figure 2. The effect of acute ethanol exposure on amygdaloid expression of histone deacetylases (HDAC) 2 and 4, and histone H3-K9 acetylation in P and NP rats
A. Representative low-magnification photomicrographs (Scale bar = 50 µm) of gold-immunolabeling of HDAC2, HDAC4, and H3-K9 acetylation in the central nucleus of amygdala (CeA) of alcohol-preferring (P) and -nonpreferring (NP) rats treated with either _n_-saline or ethanol (1 g/kg). B. HDAC2 protein levels, but not HDAC4 protein levels, were innately higher in the CeA (Group × treatment, F1, 20 = 64.8, p<0.001) and MeA (Group × treatment, F1, 20 = 84.2, p<0.001), but not BLA, of P rats in comparison to NP rats. Acute ethanol exposure reduced HDAC2 expression, but did not modulate HDAC4 expression, in the CeA and MeA of P rats, but not NP rats. Histone H3-K9 acetylation was also significantly different between the groups in the CeA and MeA, but not BLA. Acetylated H3-K9 levels were lower in the CeA (Group × treatment, F1, 20 = 104.7, p<0.001) and MeA (Group × treatment, F1, 20 = 101.7, p<0.001), but not BLA, of P rats in comparison to NP rats at baseline. Treatment with acute ethanol increased levels of histone H3-K9 acetylation in the CeA and MeA of P rats, but not NP rats. Values are represented as the mean ± SEM of the number of immunogold particles per 100 µm2 area from 6 rats per group. *Significantly different from their respective control groups (p<0.001; two-way ANOVA followed by post hoc analysis by Tukey’s test). C. Chromatin Immunoprecipitation (ChIP) analysis identified significant differences between the groups for acetylated histone H3-K9/14 associated BDNF exon IV and Arc, but not BDNF exon I (BDNF exon IV: group × treatment, F1, 20 = 8.6, p<0.01; Arc: group × treatment F1, 20 = 14.8, p<0.001). The levels of acetylated histone H3-associated gene promoter of BDNF exon IV and Arc, but not BDNF exon I, were lower in tissue homogenates composed primarily of CeA and MeA in P rats than in NP rats at baseline. Treatment with acute ethanol (1 g/kg) increased amygdaloid levels of acetylated histone H3 associated BDNF exon IV and Arc, but not BDNF exon I, in P rats, but not NP rats. Values are represented as the mean ± SEM of the fold change of acetylated histone levels associated with the aforementioned genes normalized to the NP + n-saline group and derived from 6 rats per group. *Significantly different from their respective control groups (p<0.01–0.001; two-way ANOVA followed by post hoc analysis by Tukey’s test).
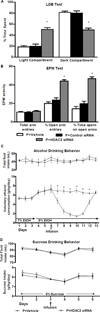
Figure 3. Effects of HDAC2 siRNA infusion into the CeA on anxiety-like behaviors and alcohol and sucrose intake in P rats
A. The light/dark box (LDB) exploration test showed that HDAC2 siRNA infusion attenuated anxiety-like behaviors of P rats in comparison to vehicle- or control siRNA-infused rats. The percentage of time spent in the light and dark compartments was significantly different among the treatment groups (F2, 36 = 36.3, p<0.001). HDAC2 siRNA infused rats spent more time in the light compartment and less time in the dark compartment than vehicle- and control siRNA-infused rats. Values are represented as the mean ± SEM of the percentage of time spent in each compartment averaged from 5–17 P rats per group. *Significantly different from control groups (p<0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test). B. The elevated plus maze (EPM) exploration test also showed that HDAC2 siRNA infusion into the CeA resulted in a reduction in the anxiety-like behaviors of P rats. P rat performance on the EPM test was significantly different between the treatment groups in percentage of open arm entries (F2,21 = 46.7, p< 0.001) and the percentage of time spent in the open arms (F2,21 = 52.2, p < 0.001). P rats infused with HDAC2 siRNA showed a higher percentage of open arm entries and time spent in the open arms than those infused with vehicle or control siRNA. The number of total arm entries does not differ significantly between the groups, suggesting that there are no effects of HDAC2 siRNA infusion on the general activity of P rats. Values represent the mean ± SEM of the percentage of open arm entries, percentage of time spent in the open arm, and number of total arm entries from 8 rats per group. *Significantly different from control groups (p<0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test). C. Voluntary ethanol consumption as measured by the two-bottle free choice paradigm was reduced by infusion of HDAC2 siRNA, but not vehicle, into the CeA of P rats. Analysis by two-way repeated measures ANOVA identified a significant difference in the amount of ethanol consumed between the treatment groups overall and daily, as indicated by the group × day interaction (Group: F1,120 = 71.9, p<0.001; Group × Day: F12,120 = 13.8, p<0.001). P rats were given access to water and 7% ethanol followed by water and 9% ethanol. Following the sixth day of ethanol access, P rats received infusion of vehicle or HDAC2 siRNA and consumption of water and 9% ethanol were monitored for several days. Total fluid intake did not significantly differ between the groups. Values are represented as the mean ± SEM (6 P rats per group) of the ethanol consumption (g/kg/day) or total fluid intake (mL) plotted daily. *Significantly different between the groups (p<0.01–0.001; post hoc analysis of the group by day interaction by Tukey’s test). D. Voluntary sucrose consumption as measured by the two-bottle free choice paradigm was unaltered by the infusion of HDAC2 siRNA and vehicle into the CeA of P rats. Analysis by two-way repeated measures ANOVA indicated no significant differences between groups. P rats were given access to water and sucrose solution as described in the methods section. Following the third day of 4% sucrose intake, P rats received infusion of vehicle or HDAC2 siRNA and consumption of water and sucrose solution were monitored for several days. Total fluid intake did not significantly differ between the groups. Values are represented as the mean ± SEM (5 P rats per group) of the sucrose intake (g/kg/day) or total fluid intake (mL) plotted daily.
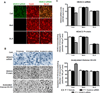
Figure 4. Effects of HDAC2 siRNA infusion into the CeA on mRNA and protein levels of HDAC2 and on histone H3-K9 acetylation in amygdaloid structures of P rats
A. Representative confocal photomicrographs reveal that HDAC2 siRNA penetrates neurons as evidenced by co-localization of fluorescence-tagged HDAC2 siRNA and neuron-specific nuclear protein (NeuN) in CeA, but not MeA or BLA. Scale bar = 50 µm. B. Representative low-magnification photomicrographs illustrating cells of in situ RT-PCR for HDAC2 mRNA and immunohistochemical staining (gold immunolabeling) for HDAC2 protein and acetylated histone H3-K9 in CeA. In comparison to vehicle and control siRNA infusion, HDAC2 siRNA infusion reduced HDAC2 mRNA and protein levels, and increased acetylated histone H3-K9 protein levels in the CeA. Scale bar = 50 µm. C. HDAC2 mRNA and protein, and histone H3-K9 acetylation levels, were significantly different between the treatment groups in the CeA, but not MeA or BLA (HDAC2 mRNA: CeA, F2, 12 = 161.0, p<0.001; HDAC2 protein: CeA, F2, 17 = 127.0, p<0.001; Acetylated histone H3-K9: CeA, F2, 17 = 70.0, p<0.001). HDAC2 mRNA and protein levels were lower in the CeA, but not MeA or BLA, of P rats infused with HDAC2 siRNA than those infused with vehicle or control siRNA. These results confirm that HDAC2 siRNA infusion causes a significant reduction in HDAC2 mRNA levels in the CeA. HDAC2 siRNA infusion into CeA also increased the levels of histone H3-K9 acetylation in the CeA, but not MeA or BLA. Values are represented as the mean ± SEM derived from 5–7 P rats per group. *Significantly different from control groups (p<0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test).
Figure 5. Effects of HDAC2 siRNA infusion into the CeA on protein levels of brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton-associated (Arc) protein and on acetylated histone H3 levels of BDNF exons I, IV and Arc genes in the amygdala of P rats
A. Representative low-magnification photomicrographs showing immunohistochemical staining (gold immunolabeling) for BDNF and Arc protein in CeA. Scale bar = 50 µm. B. Analysis of BDNF and Arc protein levels identified a significant difference between the vehicle-, HDAC2 siRNA-, and control siRNA-infused P rat groups in the CeA, but not MeA or BLA (BDNF: CeA, F2,11 = 34.5, p<0.001; Arc: CeA, F2,14 = 57.8, p<0.001). HDAC2 siRNA infusion into CeA resulted in an increase of BDNF and Arc protein in the CeA, but not MeA or BLA, of P rats in comparison to those infused with vehicle or control siRNA. Values (number of the immunogold particles per 100 µm2 area) are represented as the mean ± SEM and derived from 4–6 rats per group. *Significantly different from control groups (p<0.001; one-way ANOVA followed by post hoc analysis by Tukey’s test). C. ChIP analysis revealed that HDAC2 siRNA infusion increased the levels of acetylated histone H3-K9&14 of BDNF exon IV and Arc, but not BDNF exon I. Values represent mean ± SEM derived from 6 P rats per group. *Significantly different from vehicle-infused P rats (p<0.001; Student’s t-test).
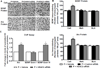
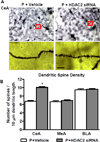
Figure 6. Effects of HDAC2 siRNA infusion into the CeA on dendritic spine density in the amygdaloid structures of P rats
A. Representative low-magnification photomicrographs (Scale bar = 50 µm) showing Golgi-impregnated neurons in the CeA of P rats infused with HDAC2 siRNA or vehicle. The boxed areas of the low magnification photographs are shown at high magnification (Scale bar = 10 µm) in adjacent photomicrographs showing dendritic spines. B. HDAC2 siRNA infusion into the CeA of P rats resulted in increased dendritic spine density in the CeA, but not MeA or BLA in comparison to vehicle-infused rats. Values (number of dendritic spines per 10 µm of dendritic length) are represented as the mean ± SEM derived from 6 rats per group. *Significantly different from control groups (p<0.001; Student’s t-test).
Figure 7
Hypothetical model of baseline differences between P and NP rats, and the effect of ethanol treatment or HDAC2 siRNA infusion into CeA with regards to chromatin structure, synaptic proteins and dendritic spines, and anxiety-like and alcohol drinking behaviors. In comparison to alcohol-nonpreferring (NP) rats, alcohol-preferring (P) rats have higher levels of histone deacetylase isoform 2 (HDAC2) in the central (CeA) and medial amygdala (MeA) resulting in deficits in histone H3-K9 acetylation. The innately higher HDAC2 levels are associated with lower histone H3 acetylation of the synaptic genes, brain-derived neurotrophic factor (BDNF) and activity-regulated cytoskeleton-associated (Arc) protein in the amygdala. This suggests that HDAC2-induced chromatin condensation, due to lower histone H3 acetylation levels, results in reduced BDNF and Arc expression and lower dendritic spine density (13) in the CeA and MeA which may play a role in anxiety-like and alcohol drinking behaviors of P rats as compared to NP rats. In P rats, the anxiolytic effects of acute ethanol exposure are associated with a reduction in HDAC2 levels in the CeA and MeA which result in increased histone H3 acetylation and relaxation of chromatin of BDNF and Arc genes. The resulting upregulation of protein levels of these genes may be responsible for the increased dendritic spine density in the CeA and MeA. Knockdown of HDAC2 via infusion of HDAC2 siRNA into the CeA results in similar effects on chromatin and synaptic remodeling, and decreases the anxiety-like and alcohol drinking behaviors of P rats. These results implicate a crucial role for HDAC2-induced chromatin and synaptic remodeling in the CeA in the regulation of anxiety-like and alcohol drinking behaviors. Ac, acetylation; Me, methylation; Ac-H3, histone H3 acetylation.
Similar articles
- Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood.
Pandey SC, Sakharkar AJ, Tang L, Zhang H. Pandey SC, et al. Neurobiol Dis. 2015 Oct;82:607-619. doi: 10.1016/j.nbd.2015.03.019. Epub 2015 Mar 24. Neurobiol Dis. 2015. PMID: 25814047 Free PMC article. - Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours.
Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, Pandey SC. Sakharkar AJ, et al. Int J Neuropsychopharmacol. 2014 Aug;17(8):1207-20. doi: 10.1017/S1461145714000054. Epub 2014 Feb 17. Int J Neuropsychopharmacol. 2014. PMID: 24528596 Free PMC article. - The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism.
Moonat S, Sakharkar AJ, Zhang H, Pandey SC. Moonat S, et al. Addict Biol. 2011 Apr;16(2):238-50. doi: 10.1111/j.1369-1600.2010.00275.x. Epub 2010 Dec 23. Addict Biol. 2011. PMID: 21182574 Free PMC article. - Epigenetic basis of the dark side of alcohol addiction.
Pandey SC, Kyzar EJ, Zhang H. Pandey SC, et al. Neuropharmacology. 2017 Aug 1;122:74-84. doi: 10.1016/j.neuropharm.2017.02.002. Epub 2017 Feb 4. Neuropharmacology. 2017. PMID: 28174112 Free PMC article. Review. - [Epigenetic mechanisms and alcohol use disorders: a potential therapeutic target].
Legastelois R, Jeanblanc J, Vilpoux C, Bourguet E, Naassila M. Legastelois R, et al. Biol Aujourdhui. 2017;211(1):83-91. doi: 10.1051/jbio/2017014. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682229 Review. French.
Cited by
- How life events may confer vulnerability to addiction: the role of epigenetics.
Liu SX, Harris AC, Gewirtz JC. Liu SX, et al. Front Mol Neurosci. 2024 Sep 18;17:1462769. doi: 10.3389/fnmol.2024.1462769. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39359689 Free PMC article. Review. - Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood.
Pandey SC, Sakharkar AJ, Tang L, Zhang H. Pandey SC, et al. Neurobiol Dis. 2015 Oct;82:607-619. doi: 10.1016/j.nbd.2015.03.019. Epub 2015 Mar 24. Neurobiol Dis. 2015. PMID: 25814047 Free PMC article. - Driving the Downward Spiral: Alcohol-Induced Dysregulation of Extended Amygdala Circuits and Negative Affect.
Centanni SW, Bedse G, Patel S, Winder DG. Centanni SW, et al. Alcohol Clin Exp Res. 2019 Oct;43(10):2000-2013. doi: 10.1111/acer.14178. Epub 2019 Aug 30. Alcohol Clin Exp Res. 2019. PMID: 31403699 Free PMC article. Review. - The histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) alleviates depression-like behavior and normalizes epigenetic changes in the hippocampus during ethanol withdrawal.
Chen WY, Zhang H, Gatta E, Glover EJ, Pandey SC, Lasek AW. Chen WY, et al. Alcohol. 2019 Aug;78:79-87. doi: 10.1016/j.alcohol.2019.02.005. Epub 2019 Mar 6. Alcohol. 2019. PMID: 30851364 Free PMC article. - Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours.
Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, Pandey SC. Sakharkar AJ, et al. Int J Neuropsychopharmacol. 2014 Aug;17(8):1207-20. doi: 10.1017/S1461145714000054. Epub 2014 Feb 17. Int J Neuropsychopharmacol. 2014. PMID: 24528596 Free PMC article.
References
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. - PubMed
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. - PubMed
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and cooccurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. - PubMed
- Bolton JM, Robinson J, Sareen J. Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. J Affect Disord. 2009;115:367–375. - PubMed
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R29 AA010005/AA/NIAAA NIH HHS/United States
- U01 AA019971/AA/NIAAA NIH HHS/United States
- AA-019971/AA/NIAAA NIH HHS/United States
- R24 AA015512/AA/NIAAA NIH HHS/United States
- AA-016690/AA/NIAAA NIH HHS/United States
- R01 AA010005/AA/NIAAA NIH HHS/United States
- R01 AA016690/AA/NIAAA NIH HHS/United States
- R24AA-015512/AA/NIAAA NIH HHS/United States
- R01 AA013341/AA/NIAAA NIH HHS/United States
- AA-013341/AA/NIAAA NIH HHS/United States
- AA-010005/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous