Acellular biomaterials: an evolving alternative to cell-based therapies - PubMed (original) (raw)
Acellular biomaterials: an evolving alternative to cell-based therapies
Jason A Burdick et al. Sci Transl Med. 2013.
Abstract
Acellular biomaterials can stimulate the local environment to repair tissues without the regulatory and scientific challenges of cell-based therapies. A greater understanding of the mechanisms of such endogenous tissue repair is furthering the design and application of these biomaterials. We discuss recent progress in acellular materials for tissue repair, using cartilage and cardiac tissues as examples of applications with substantial intrinsic hurdles, but where human translation is now occurring.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
Fig. 1. The evolving roles of acellular biomaterials in tissue repair
The application of acellular biomaterials ranges from traditional approaches of promoting tissue formation through mechanical support or the delivery of growth factors, to current/ongoing approaches to vascularize and organize tissues, and to emerging techniques that modulate endogenous cellular processes and recruitment.
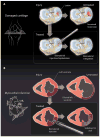
Fig. 2. Cartilage and cardiac tissues exemplify advances in acellular materials
Tissues damaged through traumatic and disease processes undergo adverse responses if left untreated. (A) In cartilage tissue, small lesions can progress to large areas of joint erosion and be accompanied by pain. Acellular, injectable gels and implantable scaffolds that recruit stem cells may improve tissue repair. In cardiac tissue, myocardial infarction initiates a cascade of events that leads to LV dilatation. (B) Acellular biomaterials can be used to alter the local stress profiles and to deliver therapeutic molecules.
Similar articles
- Heart regeneration after myocardial infarction using synthetic biomaterials.
Pascual-Gil S, Garbayo E, Díaz-Herráez P, Prosper F, Blanco-Prieto MJ. Pascual-Gil S, et al. J Control Release. 2015 Apr 10;203:23-38. doi: 10.1016/j.jconrel.2015.02.009. Epub 2015 Feb 7. J Control Release. 2015. PMID: 25665866 Review. - Injectable cardiac tissue engineering for the treatment of myocardial infarction.
Wang H, Zhou J, Liu Z, Wang C. Wang H, et al. J Cell Mol Med. 2010 May;14(5):1044-55. doi: 10.1111/j.1582-4934.2010.01046.x. Epub 2010 Feb 27. J Cell Mol Med. 2010. PMID: 20193036 Free PMC article. Review. - Cardiac tissue engineering for myocardial infarction treatment.
Gil-Cabrerizo P, Scacchetti I, Garbayo E, Blanco-Prieto MJ. Gil-Cabrerizo P, et al. Eur J Pharm Sci. 2023 Jun 1;185:106439. doi: 10.1016/j.ejps.2023.106439. Epub 2023 Mar 30. Eur J Pharm Sci. 2023. PMID: 37003408 Review. - Biomaterials for the treatment of myocardial infarction.
Christman KL, Lee RJ. Christman KL, et al. J Am Coll Cardiol. 2006 Sep 5;48(5):907-13. doi: 10.1016/j.jacc.2006.06.005. Epub 2006 Aug 17. J Am Coll Cardiol. 2006. PMID: 16949479 Review. - Advances in natural biomaterials for nerve tissue repair.
Khaing ZZ, Schmidt CE. Khaing ZZ, et al. Neurosci Lett. 2012 Jun 25;519(2):103-14. doi: 10.1016/j.neulet.2012.02.027. Epub 2012 Feb 16. Neurosci Lett. 2012. PMID: 22366403 Review.
Cited by
- A novel decellularized matrix of Wnt signaling-activated osteocytes accelerates the repair of critical-sized parietal bone defects with osteoclastogenesis, angiogenesis, and neurogenesis.
Wang X, Ma Y, Chen J, Liu Y, Liu G, Wang P, Wang B, Taketo MM, Bellido T, Tu X. Wang X, et al. Bioact Mater. 2022 Aug 16;21:110-128. doi: 10.1016/j.bioactmat.2022.07.017. eCollection 2023 Mar. Bioact Mater. 2022. PMID: 36093329 Free PMC article. - In vivo performance of an acellular disc-like angle ply structure (DAPS) for total disc replacement in a small animal model.
Martin JT, Kim DH, Milby AH, Pfeifer CG, Smith LJ, Elliott DM, Smith HE, Mauck RL. Martin JT, et al. J Orthop Res. 2017 Jan;35(1):23-31. doi: 10.1002/jor.23310. Epub 2016 Jun 14. J Orthop Res. 2017. PMID: 27227357 Free PMC article. - Personalized composite scaffolds for accelerated cell- and growth factor-free craniofacial bone regeneration.
Kim M, Wang X, Li Y, Lin Z, Collins CP, Liu Y, Ahn Y, Tsal HM, Song JW, Duan C, Zhu Y, Sun C, He TC, Luo Y, Reid RR, Ameer GA. Kim M, et al. Bioact Mater. 2024 Aug 1;41:427-439. doi: 10.1016/j.bioactmat.2024.07.029. eCollection 2024 Nov. Bioact Mater. 2024. PMID: 39188380 Free PMC article. - Epigenetic reprogramming enhances the therapeutic efficacy of osteoblast-derived extracellular vesicles to promote human bone marrow stem cell osteogenic differentiation.
Man K, Brunet MY, Fernandez-Rhodes M, Williams S, Heaney LM, Gethings LA, Federici A, Davies OG, Hoey D, Cox SC. Man K, et al. J Extracell Vesicles. 2021 Jul;10(9):e12118. doi: 10.1002/jev2.12118. Epub 2021 Jul 7. J Extracell Vesicles. 2021. PMID: 34262674 Free PMC article. - Bioactive Microsphere-Based Scaffolds Containing Decellularized Cartilage.
Sutherland AJ, Detamore MS. Sutherland AJ, et al. Macromol Biosci. 2015 Jul;15(7):979-89. doi: 10.1002/mabi.201400472. Epub 2015 Mar 27. Macromol Biosci. 2015. PMID: 25821206 Free PMC article.
References
- Mummery CL, Davis RP, Krieger JE. Challenges in using stem cells for cardiac repair. Sci Transl Med. 2010;2:27ps17. - PubMed
- McAllister TN, Dusserre N, Maruszewski M, L’Heureux N. Cell-based therapeutics from an economic perspective: Primed for a commercial success or a research sinkhole? Regen Med. 2008;3:925–937. - PubMed
- Glassman SD, Carreon LY, Campbell MJ, Johnson JR, Puno RM, Djurasovic M, Dimar JR. The perioperative cost of Infuse bone graft in posterolateral lumbar spine fusion. Spine J. 2008;8:443–448. - PubMed
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources