Learning and reconsolidation implicate different synaptic mechanisms - PubMed (original) (raw)
Learning and reconsolidation implicate different synaptic mechanisms
Yan Li et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Synaptic mechanisms underlying memory reconsolidation after retrieval are largely unknown. Here we report that synapses in projections to the lateral nucleus of the amygdala implicated in auditory fear conditioning, which are potentiated by learning, enter a labile state after memory reactivation, and must be restabilized through a postsynaptic mechanism implicating the mammalian target of rapamycin kinase-dependent signaling. Fear-conditioning-induced synaptic enhancements were primarily presynaptic in origin. Reconsolidation blockade with rapamycin, inhibiting mammalian target of rapamycin kinase activity, suppressed synaptic potentiation in slices from fear-conditioned rats. Surprisingly, this reduction of synaptic efficacy was mediated by post- but not presynaptic mechanisms. These findings suggest that different plasticity rules may apply to the processes underlying the acquisition of original fear memory and postreactivational stabilization of fear-conditioning-induced synaptic enhancements mediating fear memory reconsolidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
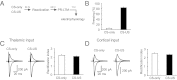
Fear conditioning leads to synaptic enhancements in cortical and thalamic inputs to the LA. (A) A schematic representation of the experimental design. Rats were trained in a single-trial fear conditioning paradigm and tested at 24 h (PR-LTM) after reactivation trials. (B) Percent freezing observed in fear-conditioned rats (CS–US, paired) and in rats that received CS or US only (CS–US, n = 22 rats; CS-only, n = 20 rats; US-only, n = 6 rats). There were no differences between freezing responses at reactivation and PR-LTM in the CS–US (P = 0.47), CS-only (P = 0.15), or US-only (P = 0.35) groups. (C) Percent freezing observed in CS–US rats at PR-LTM1 (a first reactivation trial) and PR-LTM2 (a second memory test performed 24 h after PR-LTM1) (n = 5 rats; paired t test, P = 0.51 for PR-LTM1 versus PR-LTM2). (D, Left) Averaged EPSCs evoked in thalamic input to the LA by presynaptic stimuli of increasing intensity in slices from naïve (10 rats), CS-only, US-only, and paired groups of rats. Traces are averages of 10 EPSCs. (D, Right) Synaptic input–output curves obtained in thalamic input to the LA (naïve, n = 26 neurons; CS-only, n = 16 neurons; US-only = 12 neurons; paired, n = 14 neurons). Peak amplitudes of the EPSCs were significantly different between naïve, CS-only, US-only, and paired groups (two-way ANOVA, P < 0.001). Post hoc Bonferroni’s simultaneous multiple comparisons revealed significant differences in the EPSC amplitudes between naïve and paired groups (P < 0.001), between CS-only and paired groups (P < 0.01), and between US-only and paired groups (P < 0.001). Thus, synaptic strength in thalamic input was enhanced in fear conditioned rats (paired group). (E) In cortical input, peak amplitudes of the EPSCs also differed significantly between naïve (n = 16), CS-only (n = 8), US-only (n = 12), and paired (n = 12) groups (two-way ANOVA, P < 0.001). EPSC amplitudes were larger in the paired group compared with either naïve (P < 0.001), CS-only (P < 0.001), or US-only group (P < 0.001; Bonferroni’s simultaneous multiple comparisons). Results are shown as means ± SEM.
Fig. 2.
Postretrieval rapamycin impairs reconsolidation of fear memory and suppresses conditioning-induced synaptic enhancements. (A) A schematic representation of the experiments where fear-conditioned rats received a postretrieval injection of rapamycin (RAP; 20 mg/kg, i.p.) or vehicle (VEH). (B) There was no significant difference in percent freezing between VEH-treated (n = 29) and RAP-treated (n = 29) rats during memory reactivation (t test, P = 0.74). The difference in freezing between reactivation and PR-LTM tests in the VEH group did not reach the level of statistical significance (P = 0.06). A significant impairment was observed in RAP rats during the PR-LTM test (see text for details). (C) Rapamycin had no effect on conditioned freezing in “nonreactivated” control rats. Rats in nonreactivation group received rapamycin or vehicle injections at 24 h postconditioning without memory reactivation and PR-LTM was tested 24 h after the injections (RAP, n = 16 rats; VEH, n = 8 rats; t test, P = 0.9 for VEH group vs. RAP group). (D, Left) Averaged EPSCs evoked in thalamic input to the LA by stimuli of increasing intensity in slices from fear-conditioned rats which received postreactivation injections of VEH or RAP. (D, Right) Synaptic input–output curves obtained in thalamic input in slices from both groups of rats (VEH, n = 12 neurons; RAP, n = 13 neurons (two-way ANOVA, P < 0.001 for VEH group versus RAP group of conditioned rats). (E) Experiments were analogous to D, but the EPSCs were recorded in cortical input to the LA (VEH, n = 12 neurons; RAP, n = 8 neurons; two-way ANOVA, P < 0.001). (F) Rapamycin or vehicle were injected at 24 h postconditioning without memory reactivation and synaptic input–output curves were obtained in thalamic input 24 h after the injections (VEH, n = 14 neurons; RAP, n = 23 neurons; two-way ANOVA, P = 0.275). (G) Experiments were analogous to F but the EPSCs were recorded in cortical input (VEH, n = 9 neurons; RAP, n = 19 neurons; two-way ANOVA, P = 0.515). Results are shown as means ± SEM.
Fig. 3.
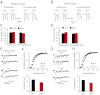
Fear-conditioning–induced synaptic strengthening in inputs to the LA is primarily presynaptically mediated. (A) A schematic representation of the experimental design. Rats were trained in a single-trial fear conditioning paradigm and tested at 24 h (PR-LTM) after reactivation trials. (B, Left) Examples of EPSCs evoked in thalamic input to the LA with paired presynaptic stimuli in slices from CS-only, US-only, and fear-conditioned (CS–US) rats. The interstimulus interval was 50 ms. Traces are averages of 10 paired EPSCs. (B, Right) Summary plot of the paired-pulse stimulation experiments. Paired pulse ratio (PPR) was calculated as the ratio of the second EPSC amplitude to the first EPSC amplitude. CS-only group of rats, n = 10 neurons; US-only group, n = 12 neurons; naïve group, n = 17 neurons; CS–US group, n = 9 neurons. The magnitude of PPR in the paired group of rats (CS–US) was significantly decreased compared with naïve, CS-only, or US-only rats (one-way ANOVA, _F_3,44 = 4.02, P = 0.013. There was no difference in PPR values between naïve and CS-only (P = 0.45) or US-only groups (P = 0.203). All electrophysiological recordings for Fig. 3 were performed at 48 h post-CS–US pairing or single CS or US presentations (24 h postreactivation). (C) Experiments were analogous to B, but the EPSCs were recorded in cortical input to the LA. CS-only group, n = 8 neurons; US-only group, n = 9 neurons; naïve group, n = 18 neurons; paired group, n = 7 neurons. The magnitude of PPR in the paired group was significantly decreased compared with naïve, CS-only, or US-only rats (one-way ANOVA, _F_3,38 = 3.37, P = 0.028). There was no difference between naïve and CS-only rats (P = 0.1) or US-only rats (P = 0.1). (D) Traces of the asynchronous quantal EPSCs evoked by stimulation of thalamic input (VH= −70 mV) in slices from the CS-only and paired rats. In these experiments, Sr2+ was substituted for extracellular Ca2+. (E, Upper) Cumulative amplitude histograms of asynchronous quantal events recorded in thalamic input to the LA in slices from the CS-only and paired groups. (E, Lower) Summary plot of asynchronous EPSCs data (mean amplitude; CS-only, n = 9 neurons; paired, n = 10 neurons; t test, P = 0.34). (F and G) Experiments were analogous to D and E, but the asynchronous EPSCs were recorded in cortical input to the LA (CS-only, n = 5 neurons; paired, n = 7 neurons; t test, P = 0.73). Error bars indicate SEM.
Fig. 4.
Rectification index for AMPAR EPSCs in inputs to the LA is not affected by single-trial fear conditioning. (A) A schematic representation of the experimental design. (B) Percent freezing observed in fear-conditioned rats (CS–US group) and CS-only rats at PR-LTM test (CS–US, n = 5 rats; CS-only, n = 6 rats; P < 0.001 between the groups). (C, Left) Averaged AMPAR EPSCs (15 traces) recorded in thalamic input to the LA at holding potentials of −70 mV, 0 mV, and +40 mV in slices from CS–US or CS-only rats. The AMPAR EPSCs were recorded in the presence of the NMDAR antagonist
D
-AP5 (50 μM). Intrapipette recording solution contained spermine (200 μM). The intensity of presynaptic stimulation was adjusted to produce the EPSCs of approximately same amplitude in both behavioral groups at a holding potential of −70 mV. (C, Right) the rectification index values at the thalamo-LA synapses in slices from CS–US and CS-only groups (CS–US group, n = 19 neurons from five rats; CS-only group, n = 23 neurons from six rats; P = 0.44 between two groups). (D) Experiments were analogous to C but the EPSCs were recorded in cortical input to the LA (CS–US group, n = 16 neurons from five rats; CS-only group, n = 22 neurons from six rats; P = 0.4 between two groups). Error bars indicate SEM.
Fig. 5.
Postretrieval stabilization of conditioning-induced potentiation in inputs to the LA implicates postsynaptic mechanisms. (A, Left) Reactivation, examples of EPSCs evoked in thalamic input to the LA with paired stimuli in slices from fear-conditioned rats that received one injection of either rapamycin (RAP; 20 mg/kg, i.p.) or vehicle (VEH) immediately after the fear memory reactivation (memory was retrieved at 24 h postconditioning). Recordings were performed 24 h after the memory reactivation. (A, Right) Nonreactivation, examples of EPSCs recorded in slices from rats that received rapamycin or vehicle injections at 24 h postconditioning without memory reactivation. Recordings were performed 24 h after the injections. (B) Analogous to A, but the EPSCs were recorded in cortical input. (C) Summary plot of PPR data in thalamic input (reactivation: VEH, n = 19 neurons; RAP, n = 21 neurons; t test, P = 0.79; nonreactivation: VEH, n = 17 neurons; RAP, n = 24 neurons; t test, P = 0.19). (D) Summary plot of PPR data in cortical input (reactivation: VEH, n = 11 neurons; RAP, n = 13 neurons; t test, P = 0.31; nonreactivation: VEH, n = 10 neurons; RAP, n = 19 neurons; t test, P = 0.63). (E) Traces of the asynchronous quantal EPSCs evoked by stimulation of thalamic input in slices from VEH or RAP groups. (F, Upper) Cumulative amplitude histograms of asynchronous quantal events recorded in thalamic input to the LA in slices from VEH or RAP rats. (F, Lower) Summary plot of asynchronous EPSCs data (mean amplitude; VEH, n = 5 neurons; RAP, n = 7 neurons; t test, *P = 0.048). (G and H) The experiments were analogous to E and F, but the asynchronous EPSCs were recorded in cortical input to the LA (VEH, n = 5 neurons; RAP, n = 6 neurons; t test, *P = 0.026). Error bars indicate SEM.
Similar articles
- Myosin light chain kinase regulates synaptic plasticity and fear learning in the lateral amygdala.
Lamprecht R, Margulies DS, Farb CR, Hou M, Johnson LR, LeDoux JE. Lamprecht R, et al. Neuroscience. 2006;139(3):821-9. doi: 10.1016/j.neuroscience.2005.12.055. Epub 2006 Mar 2. Neuroscience. 2006. PMID: 16515842 - Reactivation of fear memory renders consolidated amygdala synapses labile.
Kim J, Song B, Hong I, Kim J, Lee J, Park S, Eom JY, Lee CJ, Lee S, Choi S. Kim J, et al. J Neurosci. 2010 Jul 14;30(28):9631-40. doi: 10.1523/JNEUROSCI.0940-10.2010. J Neurosci. 2010. PMID: 20631192 Free PMC article. - Systemic inhibition of mTOR kinase via rapamycin disrupts consolidation and reconsolidation of auditory fear memory.
Mac Callum PE, Hebert M, Adamec RE, Blundell J. Mac Callum PE, et al. Neurobiol Learn Mem. 2014 Jul;112:176-85. doi: 10.1016/j.nlm.2013.08.014. Epub 2013 Sep 5. Neurobiol Learn Mem. 2014. PMID: 24012802 - Molecular mechanisms controlling protein synthesis in memory reconsolidation.
Roesler R. Roesler R. Neurobiol Learn Mem. 2017 Jul;142(Pt A):30-40. doi: 10.1016/j.nlm.2017.04.015. Epub 2017 May 2. Neurobiol Learn Mem. 2017. PMID: 28476650 Review. - Endocannabinoid signaling and memory dynamics: A synaptic perspective.
Drumond A, Madeira N, Fonseca R. Drumond A, et al. Neurobiol Learn Mem. 2017 Feb;138:62-77. doi: 10.1016/j.nlm.2016.07.031. Epub 2016 Jul 30. Neurobiol Learn Mem. 2017. PMID: 27481224 Review.
Cited by
- Electroconvulsive Shock Does Not Impair the Reconsolidation of Cued and Contextual Pavlovian Threat Memory.
Elahi H, Hong V, Ploski JE. Elahi H, et al. Int J Mol Sci. 2020 Sep 25;21(19):7072. doi: 10.3390/ijms21197072. Int J Mol Sci. 2020. PMID: 32992904 Free PMC article. - Propranolol decreases retention of fear memory by modulating the stability of surface glutamate receptor GluA1 subunits in the lateral amygdala.
Zhou J, Luo Y, Zhang JT, Li MX, Wang CM, Guan XL, Wu PF, Hu ZL, Jin Y, Ni L, Wang F, Chen JG. Zhou J, et al. Br J Pharmacol. 2015 Nov;172(21):5068-82. doi: 10.1111/bph.13272. Epub 2015 Oct 23. Br J Pharmacol. 2015. PMID: 26228348 Free PMC article. - Persistent long-term facilitation at an identified synapse becomes labile with activation of short-term heterosynaptic plasticity.
Hu JY, Schacher S. Hu JY, et al. J Neurosci. 2014 Apr 2;34(14):4776-85. doi: 10.1523/JNEUROSCI.0098-14.2014. J Neurosci. 2014. PMID: 24695698 Free PMC article. - Learning structure of sensory inputs with synaptic plasticity leads to interference.
Chrol-Cannon J, Jin Y. Chrol-Cannon J, et al. Front Comput Neurosci. 2015 Aug 5;9:103. doi: 10.3389/fncom.2015.00103. eCollection 2015. Front Comput Neurosci. 2015. PMID: 26300769 Free PMC article. - Acute and long-lasting effects of oxytocin in cortico-limbic circuits: consequences for fear recall and extinction.
Triana-Del Río R, van den Burg E, Stoop R, Hegoburu C. Triana-Del Río R, et al. Psychopharmacology (Berl). 2019 Jan;236(1):339-354. doi: 10.1007/s00213-018-5030-5. Epub 2018 Oct 9. Psychopharmacology (Berl). 2019. PMID: 30302511 Review.
References
- McGaugh JL. Memory—a century of consolidation. Science. 2000;287(5451):248–251. - PubMed
- Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. - PubMed
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406(6797):722–726. - PubMed
- Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36(3):521–525. - PubMed
- Dudai Y. Reconsolidation: The advantage of being refocused. Curr Opin Neurobiol. 2006;16(2):174–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources