Blockade of Tau hyperphosphorylation and Aβ₁₋₄₂ generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and σ₁ receptor agonist, in a nontransgenic mouse model of Alzheimer's disease - PubMed (original) (raw)
Blockade of Tau hyperphosphorylation and Aβ₁₋₄₂ generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and σ₁ receptor agonist, in a nontransgenic mouse model of Alzheimer's disease
Valentine Lahmy et al. Neuropsychopharmacology. 2013 Aug.
Abstract
The main objective of the present study was to establish whether the mixed σ₁/muscarinic ligand ANAVEX2-73, shown to be neuroprotective in Alzheimer's disease (AD) models in vivo and currently in clinical phase I/IIa, could have the ability to reduce the appearance of hyperphosphorylated Tau and amyloid-β₁₋₄₂ (Aβ₁₋₄₂ in the Aβ₂₅₋₃₅ mouse model of AD. We therefore first confirmed that Aβ₂₅₋₃₅ injection induced hyperphosphorylation of Tau protein, by showing that it rapidly decreased Akt activity and activated glycogen synthase kinase-3β (GSK-3β) in the mouse hippocampus. Second, we showed that the kinase activation, and resulting Tau alteration, directly contributed to the amyloid toxicity, as co-administration of the selective GSK-3β inhibitor 2-thio(3-iodobenzyl)-5-(1-pyridyl)-[1,3,4]-oxidiazole blocked both Tau phosphorylation and Aβ₂₅₋₃₅-induced memory impairments. Third, we analyzed the ANAVEX2-73 effect on Tau phosphorylation and activation of the related kinase pathways (Akt and GSK-3β). And fourth, we also addressed the impact of the drug on Aβ₂₅₋₃₅-induced Aβ₁₋₄₂ seeding and observed that the compound significantly blocked the increase in Aβ₁₋₄₂ and C99 levels in the hippocampus, suggesting that it may alleviate amyloid load in AD models. The comparison with PRE-084, a selective and reference σ₁ receptor agonist, and xanomeline, a muscarinic ligand presenting similar profile as ANAVEX2-73 on M1 and M2 subtypes, confirmed that both muscarinic and σ₁ targets are involved in the ANAVEX2-73 effects. The drug, acting synergistically on both targets, but with moderate affinity, presents a promising pharmacological profile.
Figures
Figure 1
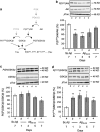
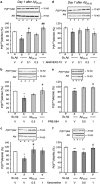
Time course of phosphorylation of Akt and GSK-3_β_ in the hippocampus of mice treated with A_β_25–35 peptide. (a) Signalling pathways involved, (b) P(S473)Akt/total Akt ratio, (c) P(S9)GSK-3_β_/total GSK-3_β_ ratio, (d) P(Y216)GSK-3_β_/total GSK-3_β_ ratio. PI3K, phospho-inositide 3-kinase; PDK1/2, protein kinase B; PYK2, proline-rich tyrosine kinase 2; Akt, serine/threonine protein kinase; GSK-3_β_, glycogen synthase kinase-3_β_. Typical blots are shown above the graphs. Lanes were from the same blot but placed in the same treatment order as shown for the graph. Mice were administered i.c.v. with Sc.A_β_ or A_β_25–35 peptide (9 nmol) and killed 1, 3, 5, 7 days after injection. ANOVA: _n_=9–15 per group, F(4,60)=4.02, p<0.01 in (b); _n_=7–12, F(4,42)=2.71, p<0.05 in (c); _n_=6–8, F(4,37)=3.23, p<0.05 in (d). *p<0.05, **p<0.01, ***p<0.001 vs the Sc.A_β_-treated group; Dunnett's test.
Figure 2
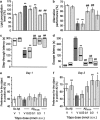
Protective effect of Tibpo against A_β_25–35-induced toxicity and learning impairments in mice. (a) Lipid peroxydation levels in the mouse hippocampus; (b) spontaneous alternation performances; (c) step-through latency; and (d) escape latency in the passive avoidance test; (e) day 1 session and (f) day 2 session in the novel object recognition test. Mice were administered with Tibpo (0.03–1 nmol i.c.v.) or the vehicle solution (V) immediately before the i.c.v. injection of scrambled A_β_25–35 or A_β_25–35 peptide (9 nmol i.c.v.) at day 0. At day 7, they were tested for spontaneous alternation, at days 8 and 9, for passive avoidance response and killed. Their hippocampus was used for lipid peroxydation measure. Another batch of animals was used at days 6–8 for the novel object recognition test in (e, f). Data show the preferential exploration index calculated as the ratio of number of contact with the object in position #2 over the total number of contacts with the two objects, expressed as percentage. The number of mice per group was _n_=6 in (a), _n_=10 in (b–d), n_=11–12 in (e, f). F(5,35)=10.9, p<0.0001 in (a); F(6,57)=5.61, p<0.001 in (b); H_=31.4, p<0.0001 in (c); H_=28.1, p<0.0001 in (d). *p<0.05, **p<0.01 vs the (Sc.A_β+V)-treated group; ##p<0.01 vs the (A_β_25–35+V)-treated group; Dunnett's test in (a, b); Dunn's test in (c, d). O_p<0.05, OO_p<0.01 vs 50% level, one-sample _t_-test in (f).
Figure 3
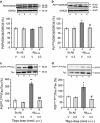
Effect of the GSK-3_β_ inhibitor Tibpo on GSK-3_β_ activity and the hyper- and abnormal phosphorylation of Tau protein 7 days after A_β_25–35 injection in mice. (a) P(Ser9)GSK-3_β_/GSK-3_β_ ratio, (b) P(Tyr216)GSK-3_β_/GSK-3_β_ ratio, (c) P(Ser202,Thr205)Tau/Tau ratio, and (d) P(Ser212, Thr214)Tau/Tau ratio. Mice were administered with Tibpo (0.3 nmol i.c.v.) or the vehicle solution (V) immediately before the i.c.v. injection of Sc.A_β_ or A_β_25–35 peptide (9 nmol i.c.v.), 7 days before killing. The number of mice per group was n_=4 in (a, b) and 10–12 in (c, d). F<1 in (a); F(3,15)=3.55, p<0.05 in (b); F(3,43)=28.1, p<0.0001 in (c); F(3,69)=4.05, p<0.05 in (d). *p<0.05, **p<0.01 vs the (Sc.A_β+V)-treated group; #p<0.05, ##p<0.01 vs the (A_β_25–35+V)-treated group; Dunnett's test.
Figure 4
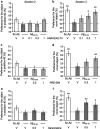
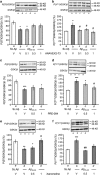
Effect of (a, b) ANAVEX2-73, (c, d) PRE-084, and (e, f) xanomeline in the novel object recognition procedure in A_β_25–35-treated mice: (a, c, e) day 1 session, (b, d, f) day 2 session. Animals were treated i.p. with ANAVEX2-73 (0.1, 0.3, 1 mg/kg), PRE-084 (0.5, 1 mg/kg), xanomeline (0.5, 1 mg/kg), or saline solution (V), 20 min before the A_β_25–35 or Sc.A_β_ (9 nmol) i.c.v. injection, at day 0. At day 6–8, they were used for the novel object recognition test. Data showed the preferential exploration index calculated as the ratio of number of contact with the object in position #2 over the total number of contacts with the two objects, expressed as percentage. n_=13–15 per group, O_p<0.05, OO_p_<0.01 vs 50% level, one-sample _t_-test.
Figure 5
Effect of ANAVEX2-73 (a, d), PRE-084 (b, e), and xanomeline (c, f) on Akt phosphorylation in the hippocampus, 1 or 7 days after A_β_25–35 injection in mice: P(S473)Akt/total Akt ratio. Mice were administered with ANAVEX2-73 (0.1–1 mg/kg i.p.), PRE-084 (0.5, 1 mg/kg i.p.), xanomeline (0.5, 1 mg/kg i.p.), or saline (V) 20 min before the A_β_25–35 or Sc.A_β_ (9 nmol) i.c.v. injection, 1 day before killing. _n_=7–8, F(4,25)=4.23, p<0.05 in (a); _n_=6–9, F(3,28)=3.39, p<0.05 in (b); _n_=4–9, F(3,25)=4.47, p<0.05 in (c); _n_=10–13, F(4,55)=3.32, p<0.05 in (d); _n_=5–13, F(3,35)=3.61, p<0.05 in (e); n_=5–9, F(3,27)=6.92, p<0.01 in (f). *p<0.05, **p<0.01 vs the (Sc.A_β+V)-treated group; #p<0.05, ##p<0.01 vs the (A_β_25–35+V)-treated group; Dunnett's test.
Figure 6
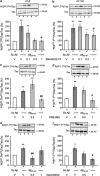
Effect of ANAVEX2-73 (a, d), PRE-084 (b, e), and xanomeline (c, f) on GSK-3_β_ phosphorylation in the hippocampus, 7 days after A_β_25–35 injection in mice. (a–c) P(Ser9)GSK-3_β_/total GSK-3_β_ ratio and (d–f) P(Tyr216)GSK-3_β_/total GSK-3_β_ ratio. Mice were administered with ANAVEX2-73 (0.1–1 mg/kg i.p.), PRE-084 (0.5, 1 mg/kg i.p.), xanomeline (0.5, 1 mg/kg i.p.), or saline (V) 20 min before the A_β_25–35 or Sc.A_β_ (9 nmol) i.c.v. injection, 7 days before killing. _n_=7–12, F(4,45)=2.81, p<0.05 in (a); _n_=5–15, F(3,41)=2.91, p<0.05 in (b); _n_=5–12, F(3,32)=5.09, p<0.01 in (c); _n_=7–9, F(4,41)=2.62, p<0.05 in (d); _n_=5–14, F(3,36)=4.28, p<0.05 in (e); n_=5–9, F(3,27)=9.51, p<0.001 in (f). *p<0.05, ** p<0.01, ***p<0.001 vs the (Sc.A_β+V)-treated group; #p<0.05, ##p<0.01 vs the (A_β_25–35+V)-treated group; Dunnett's test.
Figure 7
Effect of ANAVEX2-73 (a, b), PRE-084 (c, d), and xanomeline (e, f) on hyper- and abnormal phosphorylation of Tau protein in the hippocampus, 7 days after A_β_25–35 injection in mice. (a, c, e) P(Ser202,Thr205)Tau/total Tau ratio and (b, d, f) P(Ser212, Thr214)Tau/total Tau ratio. Mice were administered with ANAVEX2-73 (0.1–1 mg/kg i.p.), PRE-084 (0.5, 1 mg/kg i.p.), xanomeline (0.5, 1 mg/kg i.p.), or saline 20 min before the A_β_25–35 or Sc.A_β_ (9 nmol) i.c.v. injection, 7 days before killing. _n_=7–14, F(4,45)=4.58, p<0.01 in (a); _n_=12–17, F(4,75)=3.59, _p_=0.01 in (b); _n_=5–7, F(3,22)=10.5, p<0.001 in (c); _n_=5–11, F(3,35)=4.14, p<0.05 in (d); _n_=5–7, F(3,24)=3.88, p<0.05 in (e); n_=4–14, F(3,36)=3.98, p<0.05 in (f). *p<0.05, **p<0.01, ***p<0.001 vs the (Sc.A_β+V)-treated group; #p<0.05, ##p<0.01 vs the (A_β_25–35+V)-treated group; Dunnett's test.
Figure 8
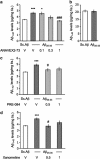
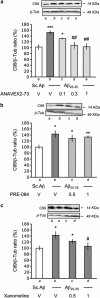
Effect of ANAVEX2-73, PRE-084, and xanomeline on A_β_1–42 and A_β_1–40 contents measured by ELISA assay in the hippocampus, 7 days after A_β_25–35 injection in mice. (a) Effect of ANAVEX2-73 on A_β_25–35-induced increase in A_β_1–42 content; (b) lack of effect of A_β_25–35 on A_β_1–40 content; (c) effect of PRE-084 on A_β_25–35-induced increase in A_β_1–42 content; (d) effect of xanomeline on A_β_25–35-induced increase in A_β_1–42 content. Mice were administered with ANAVEX2-73 (0.1–1 mg/kg i.p.), PRE-084 (0.5, 1 mg/kg i.p.), xanomeline (0.5, 1 mg/kg i.p.), or saline (V) 20 min before the A_β_25–35 or Sc.A_β_ (9 nmol) i.c.v. injection, 7 days before killing. _n_=6–9, F(4,31)=7.43, p<0.05 in (a); _n_=6, _t_=0.12, _p_>0.05 in (b); _n_=6–7, F(3,24)=4.58, p<0.05 in (c); n_=6, F(3,23)=6.65, p<0.01 in (d). *p<0.05, ** p<0.01, ***p<0.001 vs the (Sc.A_β+V)-treated group; #p<0.05, ###p<0.001 vs the (A_β_25–35+V)-treated group; Dunnett's test.
Figure 9
Effect of ANAVEX2-73 (a), PRE-084 (b), and xanomeline (c) on amyloid C99 fragment level in the hippocampus, 7 days after A_β_25–35 injection in mice. Mice were administered with ANAVEX2-73 (0.1–1 mg/kg i.p.), PRE-084 (0.5, 1 mg/kg i.p.), xanomeline (0.5, 1 mg/kg i.p.), or saline (V) 20 min before the A_β_25–35 or Sc.A_β_ (9 nmol) i.c.v. injection, 7 days before killing. _n_=6–8, F(4,33)=6.50, p<0.001 in (a); _n_=10–12, F(3,20)=3.47, p<0.05 in (b); n_=10–12, F(3,21)=3.59, p<0.05 in (c). *p<0.05, ***p<0.001 vs the (Sc.A_β+V)-treated group; #p<0.05, ##p<0.01 vs the (A_β_25–35+V)-treated group; Dunnett's test.
Similar articles
- Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 (σ1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative.
Villard V, Espallergues J, Keller E, Vamvakides A, Maurice T. Villard V, et al. J Psychopharmacol. 2011 Aug;25(8):1101-17. doi: 10.1177/0269881110379286. Epub 2010 Sep 9. J Psychopharmacol. 2011. PMID: 20829307 - FLZ alleviates the memory deficits in transgenic mouse model of Alzheimer's disease via decreasing beta-amyloid production and tau hyperphosphorylation.
Bao XQ, Li N, Wang T, Kong XC, Tai WJ, Sun H, Zhang D. Bao XQ, et al. PLoS One. 2013 Nov 4;8(11):e78033. doi: 10.1371/journal.pone.0078033. eCollection 2013. PLoS One. 2013. PMID: 24223757 Free PMC article. - Arctigenin Attenuates Learning and Memory Deficits through PI3k/Akt/GSK-3β Pathway Reducing Tau Hyperphosphorylation in Aβ-Induced AD Mice.
Qi Y, Dou DQ, Jiang H, Zhang BB, Qin WY, Kang K, Zhang N, Jia D. Qi Y, et al. Planta Med. 2017 Jan;83(1-02):51-56. doi: 10.1055/s-0042-107471. Epub 2016 May 25. Planta Med. 2017. PMID: 27224270 - M1 muscarinic agonists can modulate some of the hallmarks in Alzheimer's disease: implications in future therapy.
Fisher A, Pittel Z, Haring R, Bar-Ner N, Kliger-Spatz M, Natan N, Egozi I, Sonego H, Marcovitch I, Brandeis R. Fisher A, et al. J Mol Neurosci. 2003;20(3):349-56. doi: 10.1385/JMN:20:3:349. J Mol Neurosci. 2003. PMID: 14501019 Review. - Therapeutic strategies in Alzheimer's disease: M1 muscarinic agonists.
Fisher A. Fisher A. Jpn J Pharmacol. 2000 Oct;84(2):101-12. doi: 10.1254/jjp.84.101. Jpn J Pharmacol. 2000. PMID: 11128032 Review.
Cited by
- Dual-Acting Cholinesterase-Human Cannabinoid Receptor 2 Ligands Show Pronounced Neuroprotection in Vitro and Overadditive and Disease-Modifying Neuroprotective Effects in Vivo.
Scheiner M, Dolles D, Gunesch S, Hoffmann M, Nabissi M, Marinelli O, Naldi M, Bartolini M, Petralla S, Poeta E, Monti B, Falkeis C, Vieth M, Hübner H, Gmeiner P, Maitra R, Maurice T, Decker M. Scheiner M, et al. J Med Chem. 2019 Oct 24;62(20):9078-9102. doi: 10.1021/acs.jmedchem.9b00623. Epub 2019 Oct 14. J Med Chem. 2019. PMID: 31609608 Free PMC article. - Revisiting the sigma-1 receptor as a biological target to treat affective and cognitive disorders.
Sałaciak K, Pytka K. Sałaciak K, et al. Neurosci Biobehav Rev. 2022 Jan;132:1114-1136. doi: 10.1016/j.neubiorev.2021.10.037. Epub 2021 Nov 1. Neurosci Biobehav Rev. 2022. PMID: 34736882 Free PMC article. Review. - Amyloid β-based therapy for Alzheimer's disease: challenges, successes and future.
Zhang Y, Chen H, Li R, Sterling K, Song W. Zhang Y, et al. Signal Transduct Target Ther. 2023 Jun 30;8(1):248. doi: 10.1038/s41392-023-01484-7. Signal Transduct Target Ther. 2023. PMID: 37386015 Free PMC article. Review. - 7-O-Esters of taxifolin with pronounced and overadditive effects in neuroprotection, anti-neuroinflammation, and amelioration of short-term memory impairment in vivo.
Gunesch S, Hoffmann M, Kiermeier C, Fischer W, Pinto AFM, Maurice T, Maher P, Decker M. Gunesch S, et al. Redox Biol. 2020 Jan;29:101378. doi: 10.1016/j.redox.2019.101378. Epub 2019 Nov 9. Redox Biol. 2020. PMID: 31926632 Free PMC article. - Therapeutic Strategies Targeting Mitochondrial Calcium Signaling: A New Hope for Neurological Diseases?
Rodríguez LR, Lapeña-Luzón T, Benetó N, Beltran-Beltran V, Pallardó FV, Gonzalez-Cabo P, Navarro JA. Rodríguez LR, et al. Antioxidants (Basel). 2022 Jan 15;11(1):165. doi: 10.3390/antiox11010165. Antioxidants (Basel). 2022. PMID: 35052668 Free PMC article. Review.
References
- Akiyama H, Shin RW, Uchida C, Kitamoto T, Uchida T. Pin1 promotes production of Alzheimer's amyloid β from β-cleaved amyloid precursor protein. Biochem Biophys Res Commun. 2005;336:521–529. - PubMed
- Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004;89:1313–1317. - PubMed
- Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical