Estimating the effect of sulfonylurea on HbA1c in diabetes: a systematic review and meta-analysis - PubMed (original) (raw)
Review
Estimating the effect of sulfonylurea on HbA1c in diabetes: a systematic review and meta-analysis
J A Hirst et al. Diabetologia. 2013 May.
Abstract
Aims/hypothesis: Sulfonylureas are widely prescribed glucose-lowering medications for diabetes, but the extent to which they improve glycaemia is poorly documented. This systematic review evaluates how sulfonylurea treatment affects glycaemic control.
Methods: Medline, EMBASE, the Cochrane Library and clinical trials registries were searched to identify double-blinded randomised controlled trials of fixed-dose sulfonylurea monotherapy or sulfonylurea added on to other glucose-lowering treatments. The primary outcome assessed was change in HbA1c, and secondary outcomes were adverse events, insulin dose and change in body weight.
Results: Thirty-one trials with a median duration of 16 weeks were included in the meta-analysis. Sulfonylurea monotherapy (nine trials) lowered HbA1c by 1.51% (17 mmol/mol) more than placebo (95% CI, 1.25, 1.78). Sulfonylureas added to oral diabetes treatment (four trials) lowered HbA1c by 1.62% (18 mmol/mol; 95% CI 1.0, 2.24) compared with the other treatment, and sulfonylurea added to insulin (17 trials) lowered HbA1c by 0.46% (6 mmol/mol; 95% CI 0.24, 0.69) and lowered insulin dose. Higher sulfonylurea doses did not reduce HbA1c more than lower doses. Sulfonylurea treatment resulted in more hypoglycaemic events (RR 2.41, 95% CI 1.41, 4.10) but did not significantly affect the number of other adverse events. Trial length, sulfonylurea type and duration of diabetes contributed to heterogeneity.
Conclusions/interpretation: Sulfonylurea monotherapy lowered HbA1c level more than previously reported, and we found no evidence that increasing sulfonylurea doses resulted in lower HbA1c. HbA1c is a surrogate endpoint, and we were unable to examine long-term endpoints in these predominately short-term trials, but sulfonylureas appear to be associated with an increased risk of hypoglycaemic events.
Figures
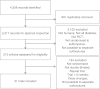
Fig. 1
Flow chart of searches
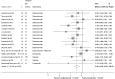
Fig. 2
Mean difference in change in HbA1c of sulfonylurea monotherapy treatment vs placebo (boxes) and pooled estimates (diamonds) calculated by the random effects DerSimonian and Laird method. Horizontal bars and diamond widths denote 95% CIs, and box sizes indicate relative weight in the analysis
Fig. 3
Mean difference in change in HbA1c of sulfonylurea treatment added on to another oral treatment vs placebo + other treatment (boxes) and pooled estimates (diamonds) calculated by the random effects DerSimonian and Laird method. Horizontal bars and diamond widths denote 95% CIs, and box sizes indicate relative weight in the analysis. Doses in parentheses are doses of troglitazone, the background therapy
Fig. 4
Mean difference in change in HbA1c of sulfonylurea treatment added on to insulin treatment vs insulin + placebo (boxes) and pooled estimates (diamonds) calculated by the random effects DerSimonian and Laird method. Horizontal bars and diamond widths denote 95% CIs, and box sizes indicate relative weight in the analysis
Fig. 5
Mean difference in change in HbA1c of higher sulfonylurea dose vs lower sulfonylurea dose (boxes) and pooled estimates (diamonds) calculated by the random effects DerSimonian and Laird method, stratified by sulfonylurea type. Horizontal bars and diamond widths denote 95% CIs, and box sizes indicate relative weight in the analysis. qd, once daily, bid, twice a day
Fig. 6
Relative risk of total adverse events, serious adverse events and hypoglycaemic events during trials of sulfonylurea treatment vs comparator (boxes) and pooled estimates across trials (diamonds) calculated by the fixed effects inverse variance (I–V) method in patients with diabetes. Horizontal bars and diamond widths denote 95% CIs, and box sizes indicate relative weight in the I–V analysis
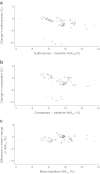
Fig. 7
(a) Sulfonylurea group−change vs baseline HbA1c. (b) Comparator group−change vs baseline HbA1c. (c) Difference in HbA1c vs mean baseline HbA1c by treatment type. Circles represent monotherapy trials, triangles represent oral therapy combination trials and diamonds represent insulin combination trials. Marker size is proportional to trial size
Similar articles
- A 24-week study to evaluate the efficacy and safety of once-weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8).
Dungan KM, Weitgasser R, Perez Manghi F, Pintilei E, Fahrbach JL, Jiang HH, Shell J, Robertson KE. Dungan KM, et al. Diabetes Obes Metab. 2016 May;18(5):475-82. doi: 10.1111/dom.12634. Epub 2016 Feb 19. Diabetes Obes Metab. 2016. PMID: 26799540 Free PMC article. Clinical Trial. - Canagliflozin in Conjunction With Sulfonylurea Maintains Glycemic Control and Weight Loss Over 52 Weeks: A Randomized, Controlled Trial in Patients With Type 2 Diabetes Mellitus.
Yale JF, Xie J, Sherman SE, Garceau C. Yale JF, et al. Clin Ther. 2017 Nov;39(11):2230-2242.e2. doi: 10.1016/j.clinthera.2017.10.003. Epub 2017 Nov 3. Clin Ther. 2017. PMID: 29103664 Clinical Trial. - Efficacy and safety of linagliptin in subjects with type 2 diabetes mellitus and poor glycemic control: pooled analysis of data from three placebo-controlled phase III trials.
Del Prato S, Taskinen MR, Owens DR, von Eynatten M, Emser A, Gong Y, Chiavetta S, Patel S, Woerle HJ. Del Prato S, et al. J Diabetes Complications. 2013 May-Jun;27(3):274-9. doi: 10.1016/j.jdiacomp.2012.11.008. Epub 2013 Feb 9. J Diabetes Complications. 2013. PMID: 23403068 Clinical Trial. - The benefits and risks of DPP4-inhibitors vs. sulfonylureas for patients with type 2 diabetes: accumulated evidence from randomised controlled trial.
Zhou JB, Bai L, Wang Y, Yang JK. Zhou JB, et al. Int J Clin Pract. 2016 Feb;70(2):132-41. doi: 10.1111/ijcp.12761. Epub 2015 Dec 28. Int J Clin Pract. 2016. PMID: 26709610 Review. - Systematic review and meta-analysis of the efficacy and hypoglycemic safety of gliclazide versus other insulinotropic agents.
Chan SP, Colagiuri S. Chan SP, et al. Diabetes Res Clin Pract. 2015 Oct;110(1):75-81. doi: 10.1016/j.diabres.2015.07.002. Epub 2015 Jul 9. Diabetes Res Clin Pract. 2015. PMID: 26361859 Review.
Cited by
- Anti-Hyperglycemic Medication Management in the Perioperative Setting: A Review and Illustrative Case of an Adverse Effect of GLP-1 Receptor Agonist.
Goron AR, Connolly C, Valdez-Sinon AN, Hesson A, Helou C, Kirschen GW. Goron AR, et al. J Clin Med. 2024 Oct 20;13(20):6259. doi: 10.3390/jcm13206259. J Clin Med. 2024. PMID: 39458209 Free PMC article. Review. - Current Position of Gliclazide and Sulfonylureas in the Contemporary Treatment Paradigm for Type 2 Diabetes: A Scoping Review.
Sahin I, Bakiner O, Demir T, Sari R, Atmaca A. Sahin I, et al. Diabetes Ther. 2024 Aug;15(8):1687-1716. doi: 10.1007/s13300-024-01612-8. Epub 2024 Jun 27. Diabetes Ther. 2024. PMID: 38935188 Free PMC article. Review. - Exploring Innovative Approaches in Type-2 Diabetes Management: A Comprehensive Review on Nano-carriers and Transdermal Drug Delivery.
Chauhan N, Kumar M, Kumar K, Chopra S, Bhatia A. Chauhan N, et al. Curr Pharm Des. 2024;30(22):1725-1745. doi: 10.2174/0113816128313325240513113840. Curr Pharm Des. 2024. PMID: 38847167 Review. - Liraglutide and Robust A1C Reductions Among People With Type 2 Diabetes Requiring Appetite Control: A Review of Two Cases.
Pitts MA, Griggs RH, Hall MR, Tankersley MS, Johnson JL. Pitts MA, et al. Diabetes Spectr. 2024 Spring;37(2):175-179. doi: 10.2337/ds23-0052. Epub 2023 Dec 14. Diabetes Spectr. 2024. PMID: 38756434 No abstract available. - Continuous glucose monitoring for the routine care of type 2 diabetes mellitus.
Ajjan RA, Battelino T, Cos X, Del Prato S, Philips JC, Meyer L, Seufert J, Seidu S. Ajjan RA, et al. Nat Rev Endocrinol. 2024 Jul;20(7):426-440. doi: 10.1038/s41574-024-00973-1. Epub 2024 Apr 8. Nat Rev Endocrinol. 2024. PMID: 38589493 Review.
References
- NICE (2008) CG66 type 2 diabetes: full guideline. In: NICE clinical guideline 66. www.nice.org.uk/nicemedia/pdf/CG66NICEGuideline.pdf
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2009;52:17–30. doi: 10.1007/s00125-008-1157-y. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical