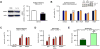G protein-coupled receptor kinase 2 and group I metabotropic glutamate receptors mediate inflammation-induced sensitization to excitotoxic neurodegeneration - PubMed (original) (raw)
doi: 10.1002/ana.23868. Epub 2013 Mar 14.
Stéphane Peineau, Cora Nijboer, Angela M Kaindl, Stéphanie Sigaut, Géraldine Favrais, Frank Plaisant, Natacha Teissier, Elodie Gouadon, Alain Lombet, Elie Saliba, Graham L Collingridge, Mervyn Maze, Ferdinando Nicoletti, Cobi Heijnen, Jean Mantz, Annemieke Kavelaars, Pierre Gressens
Affiliations
- PMID: 23494575
- PMCID: PMC3837433
- DOI: 10.1002/ana.23868
G protein-coupled receptor kinase 2 and group I metabotropic glutamate receptors mediate inflammation-induced sensitization to excitotoxic neurodegeneration
Vincent Degos et al. Ann Neurol. 2013 May.
Abstract
Objective: The concept of inflammation-induced sensitization is emerging in the field of perinatal brain injury, stroke, Alzheimer disease, and multiple sclerosis. However, mechanisms underpinning this process remain unidentified.
Methods: We combined in vivo systemic lipopolysaccharide-induced or interleukin (IL)-1β-induced sensitization of neonatal and adult rodent cortical neurons to excitotoxic neurodegeneration with in vitro IL-1β sensitization of human and rodent neurons to excitotoxic neurodegeneration. Within these inflammation-induced sensitization models, we assessed metabotropic glutamate receptors (mGluR) signaling and regulation.
Results: We demonstrate for the first time that group I mGluRs mediate inflammation-induced sensitization to neuronal excitotoxicity in neonatal and adult neurons across species. Inflammation-induced G protein-coupled receptor kinase 2 (GRK2) downregulation and genetic deletion of GRK2 mimicked the sensitizing effect of inflammation on excitotoxic neurodegeneration. Thus, we identify GRK2 as a potential molecular link between inflammation and mGluR-mediated sensitization.
Interpretation: Collectively, our findings indicate that inflammation-induced sensitization is universal across species and ages and that group I mGluRs and GRK2 represent new avenues for neuroprotection in perinatal and adult neurological disorders.
Copyright © 2013 American Neurological Association.
Figures
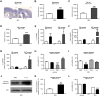
Figure 1. Inflammation sensitizes neurons to excitotoxic neurodegeneration in vivo and in vitro
A. Cresyl violet-stained sections showing lesions induced by ibotenate (IBO) injected on P5 following PBS or IL-1β injection between P1 and P5. Bar=80µm. B. Quantification of cortical plate lesions induced by ibotenate in P5 mice following PBS (white bar) or IL-1β (black bar) injections between P1 and P5 (n=16-28/group). C. Quantification of cortical plate lesions induced by ibotenate in P5 rats following PBS (white bar) or LPS (hatched bar) injections between E19 and E20 (n=12-16/group). D. Quantification of cortical plate lesions induced by ibotenate in P47 mice following PBS (white bar) or LPS (hatched bar) injections between P45 and P47 (n=9-15/group). E-G. qRT-PCR of the cortical mRNA levels of IL-1β (E), TNF-α (F), and IL-6 **(**G) in mice treated between P1 and P5 with PBS (white bar) or IL-1β (black bar) (n=14-22/group), and in mice treated between P45 and P47 with PBS (white bar) or LPS (hatched bar) (n=6/group). H. Cell viability quantification in mouse neurons cultured with PBS, IL-1β, TNFα, or IL-6 (n=30-40 wells/group). I. Cell viability quantification in mouse neurons cultured with PBS, IL-1β, TNFα, or IL-6 and exposed to ibotenate on the fourth day (n=12 wells/group). J-K. Western blot for cleaved caspase-3 in mouse neurons cultured with PBS (white bar) or IL-1β (black bar) and exposed to ibotenate on the fourth day (n=6 wells/group). L. Cell viability quantification in human neurons cultured with PBS (white bar) or IL-1β (black bar) and exposed to vehicle or ibotenate on the fifth day (n=12 wells/group). Bars represent mean + SEM. * p<0.05, ** p<0.01, *** p<0.001 in Student's _t_-test (B-G, K-L) or ANOVA with Bonferroni's multiple comparison tests (H-I). PBS: Phosphate buffered saline; IL-1β: interleukin-1β; IBO: ibotenate; LPS: lipopolysaccharide.
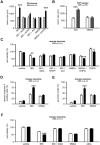
Figure 2. Sensitization requires the activation of group I mGluR in vivo and in vitro
A. Quantification of cortical plate lesions induced by glutamate analogues (indicated on the X axis) in P5 mice following PBS (white bar) or IL-1β (black bar) injections between P1 and P5 (n=12-28/group). B. Quantification of cortical plate lesions induced by glutamate analogues (indicated on the X axis) in P47 mice following PBS (white bar) or LPS (hatched bar) injections between P45 and P47 (n=10-12/group). C-E. Quantification of cell viability (C), western blot for cleaved caspase-3 (D) and pycnotic nuclei (E) in mouse neurons cultured in the presence of PBS (white bars) or IL-1β (black bars) and exposed to glutamate analogues (indicated on the X axis) on the fourth day (n=10-18 well/group). F. Quantification of cell viability in human neurons cultured in the presence of PBS (white bar) or IL-1β (black bar) and exposed to glutamate analogues (indicated on the X axis) on the fifth day (n=8 wells/group). Bars represent mean + SEM. * p<0.05, ** p<0.01, *** p<0.001 in ANOVA with Bonferroni's multiple comparison tests vs. PBS-injected mice (A-B) and vs PBS-treated neurons (C-F). PBS: Phosphate buffered saline; IL-1β: interleukin-1β; LPS: lipopolysaccharide; IBO: ibotenate; AIDA: 1-aminoindan-1,5-dicarboxylic acid (group I mGluR antagonist); MTEP: 3-((2-Methyl-1,3-thiazol-4-yl)ethynyl)pyridine hydrochloride (mGlu5 negative allosteric modulator); JNJ: JNJ16259685, 3,4-dihydro-2_H_-pyrano[2,3-_b_]quinolin-7-yl)-(_cis_-4-methoxycyclohexyl)-methanone, (mGlu1 negative allosteric modulator); NMDA: N-methyl-D-aspartate, DHPG: 3,5-dihydroxyphenylglycin, (group I mGluR agonist).
Figure 3. Effect of inflammation on the metabotropic glutamatergic receptor expression
A. qRT-PCR of mGluRs in mouse neurons cultured in the presence of PBS (white bars) or IL-1β (black bars; 50ng/ml once a day for 4 days) and exposed to ibotenate on the fourth day (n=10 wells/group). B-C. Quantification of western blot for mGlu1α (C) and mGlu5 (D) in mouse neurons cultured in the presence of PBS (white bars) or IL-1β (black bars; 50 ng/ml once a day for 4 days) and exposed to ibotenate on the fourth day (n=12 wells/group). Bars represent mean + SEM. PBS: Phosphate buffered saline; IL-1β: interleukin-1β.
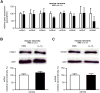
Figure 4. Inflammation exacerbates ibotenate-induced calcium mobilization in neurons
A. Quantification of cortical plate lesions induced by ibotenate+vehicle or ibotenate+U73122 (PLCβ1 inhibitor) on P5 following PBS (white bar) or IL-1β (black bar) between P1 and P5 (n=8-10/group). B. Quantification of cortical plate lesions induced by ibotenate+vehicle or ibotenate+U73122 on P47 following PBS (white bar) or LPS (hatched bar) between P45 and P47 (n=12/group). C. Quantification of cell viability in mouse neurons cultured in the presence of PBS (white bar) or IL-1β (black bar) and exposed to vehicle, ibotenate+vehicle or ibotenate+transduction inhibitors (b-indo, bis-indolylmaleimide; chele, chelerythrine) on the fourth day (n=12-18 wells/group). D. Quantification of western blot for PLCβ1 in mouse neurons cultured with PBS (white bars) or IL-1β (black bars) (n=8 wells/group). E. Representative images and traces of calcium levels in mouse neurons cultured with PBS (black trace) or IL-1β (red trace) and exposed on the fourth day to vehicle (a) prior to 100μM ibotenate (b), followed by vehicle wash (c). Bar=50μm. F-G. Quantification of the area under the curve (F) and maximal amplitude (G) of calcium levels in mouse neurons cultured with PBS (white bar) or IL-1β (black bar) and exposed to different concentrations of ibotenate (n=35-50 wells/group). The scale indicates Ca2+ level, expressed as ΔR/R values, where R is the ratio (R) between fluorescence signals at 340 and 380 nm obtained before the addition of any agent, and ΔR the difference between the ratios measured during a response and R. Bars represent mean + SEM. * p<0.05, *** p<0.001 in ANOVA with Bonferroni's multiple comparison tests vs. PBS-injected mice (A-B) and vs PBS-treated neurons (C-G). PBS: Phosphate buffered saline; IL-1β: interleukin-1β; LPS: lipopolysaccharide; IBO: ibotenate; PLCβ1: Phospholipase Cβ1.
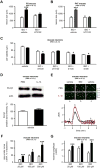
Figure 5. Role of GRK2 in the sensitizing effect of inflammation
A. Quantification of western blot for GRK2 in human neurons cultured in the presence of PBS (white bar) or IL-1β (black bar) (n=24 wells/group). B. Quantification of cell viability in human neurons transfected with MOCK vector (blue bar) or with _GRK2_-expressing vector (orange bar) and exposed to glutamate analogues (indicated on the X axis) on the fifth day (n=8 wells/group). C. Quantification of cell viability in mouse neurons from GRK2+/+ (red bars) and GRK2+/- (pink bars) mice exposed to vehicle, ibotenate or NMDA (n=15-18 wells/group). D. Quantification of the cortical plate size of brain lesions induced by glutamate analogues (indicated on the X axis) on P5 and studied on P10 in GRK2+/+ (red bars) and GRK2+/- (pink bars) mice (n=7-9/group). E. Quantification of the cortical plate size of brain lesions induced by ibotenate on P5 and studied on P10 in GRK2+/+CamK2aCre/+(dark green bar) and GRK2flox/+CamK2aCre/+(light green bar) mice (n=7/group). Bars represent mean + SEM. * p<0.05, ** p<0.01, *** p<0.001 in Student's _t_-test (A, E) or in ANOVA with Bonferroni's multiple comparison tests vs. MOCK-transferred neurons (B) and vs. GRK2+/+ mice (C-D) . PBS: Phosphate buffered saline; IL-1β: interleukin-1β; IBO: ibotenate; AIDA: 1-aminoindan-1,5-dicarboxylic acid (group I mGluR antagonist); NMDA: N-methyl-D-aspartate; DHPG: 3,5-dihydroxyphenylglycine.
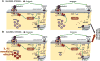
Figure 6. Schematic representation of the potential mechanisms by which group 1 mGluR and GRK2 mediate IL-1β-induced sensitization
The diagram depicts the molecular mechanisms leading to excitotoxic neuronal cell death in the absence (A) or presence (B) of IL-1β sensitizing effect. In the absence of IL-1β, GRK2 leads to a rapid desensitization of mGlu1/5, limiting the PLCβ1-mediated calcium release from endogenous stores. Exposure to IL-1β leads to a reduced content of GRK2, preventing the complete desensitization of mGlu1/5 and allowing a more prolonged calcium release from endogenous stores. This enhanced calcium mobilization exacerbates neuronal cell death. E.R., endoplasmic reticulum.
Similar articles
- Neuroinflammation is associated with changes in glial mGluR5 expression and the development of neonatal excitotoxic lesions.
Drouin-Ouellet J, Brownell AL, Saint-Pierre M, Fasano C, Emond V, Trudeau LE, Lévesque D, Cicchetti F. Drouin-Ouellet J, et al. Glia. 2011 Feb;59(2):188-99. doi: 10.1002/glia.21086. Glia. 2011. PMID: 21125661 Free PMC article. - Phosphorylation-independent regulation of metabotropic glutamate receptor 5 desensitization and internalization by G protein-coupled receptor kinase 2 in neurons.
Ribeiro FM, Ferreira LT, Paquet M, Cregan T, Ding Q, Gros R, Ferguson SS. Ribeiro FM, et al. J Biol Chem. 2009 Aug 28;284(35):23444-53. doi: 10.1074/jbc.M109.000778. Epub 2009 Jun 29. J Biol Chem. 2009. PMID: 19564331 Free PMC article. - Evidence against a permissive role of the metabotropic glutamate receptor 1 in acute excitotoxicity.
Ferraguti F, Pietra C, Valerio E, Corti C, Chiamulera C, Conquet F. Ferraguti F, et al. Neuroscience. 1997 Jul;79(1):1-5. doi: 10.1016/s0306-4522(97)00074-2. Neuroscience. 1997. PMID: 9178862 - Metabotropic glutamate receptors as targets for multipotential treatment of neurological disorders.
Byrnes KR, Loane DJ, Faden AI. Byrnes KR, et al. Neurotherapeutics. 2009 Jan;6(1):94-107. doi: 10.1016/j.nurt.2008.10.038. Neurotherapeutics. 2009. PMID: 19110202 Free PMC article. Review. - Inflammation-induced sensitization of the brain in term infants.
Fleiss B, Tann CJ, Degos V, Sigaut S, Van Steenwinckel J, Schang AL, Kichev A, Robertson NJ, Mallard C, Hagberg H, Gressens P. Fleiss B, et al. Dev Med Child Neurol. 2015 Apr;57 Suppl 3:17-28. doi: 10.1111/dmcn.12723. Dev Med Child Neurol. 2015. PMID: 25800488 Review.
Cited by
- Proinflammatory Cytokines Mediate GPCR Dysfunction.
Mohan ML, Vasudevan NT, Naga Prasad SV. Mohan ML, et al. J Cardiovasc Pharmacol. 2017 Aug;70(2):61-73. doi: 10.1097/FJC.0000000000000456. J Cardiovasc Pharmacol. 2017. PMID: 28763371 Free PMC article. Review. - AgRP/NPY Neuron Excitability Is Modulated by Metabotropic Glutamate Receptor 1 During Fasting.
Laing BT, Li P, Schmidt CA, Bunner W, Yuan Y, Landry T, Prete A, McClung JM, Huang H. Laing BT, et al. Front Cell Neurosci. 2018 Sep 3;12:276. doi: 10.3389/fncel.2018.00276. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30233321 Free PMC article. - Administration of Bacterial Lipopolysaccharide during Early Postnatal Ontogenesis Induces Transient Impairment of Long-Term Synaptic Plasticity Associated with Behavioral Abnormalities in Young Rats.
Postnikova TY, Griflyuk AV, Ergina JL, Zubareva OE, Zaitsev AV. Postnikova TY, et al. Pharmaceuticals (Basel). 2020 Mar 18;13(3):48. doi: 10.3390/ph13030048. Pharmaceuticals (Basel). 2020. PMID: 32197321 Free PMC article. - G protein-coupled receptors in neurodegenerative diseases and psychiatric disorders.
Wong TS, Li G, Li S, Gao W, Chen G, Gan S, Zhang M, Li H, Wu S, Du Y. Wong TS, et al. Signal Transduct Target Ther. 2023 May 3;8(1):177. doi: 10.1038/s41392-023-01427-2. Signal Transduct Target Ther. 2023. PMID: 37137892 Free PMC article. Review. - mGlu3 receptor regulates microglial cell reactivity in neonatal rats.
Zinni M, Mairesse J, Pansiot J, Fazio F, Iacovelli L, Antenucci N, Orlando R, Nicoletti F, Vaiman D, Baud O. Zinni M, et al. J Neuroinflammation. 2021 Jan 6;18(1):13. doi: 10.1186/s12974-020-02049-z. J Neuroinflammation. 2021. PMID: 33407565 Free PMC article.
References
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. - PubMed
- Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–808. - PubMed
- Degos V, Favrais G, Kaindl AM, et al. Inflammation processes in perinatal brain damage. J Neural Transm. 2010;117:1009–1017. - PubMed
- Dommergues MA, Patkai J, Renauld JC, et al. Proinflammatory cytokines and interleukin-9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol. 2000;47:54–63. - PubMed
- McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158:1049–1061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS074999/NS/NINDS NIH HHS/United States
- 089703/WT_/Wellcome Trust/United Kingdom
- NS073939/NS/NINDS NIH HHS/United States
- R01 NS073939/NS/NINDS NIH HHS/United States
- NS074999/NS/NINDS NIH HHS/United States
- MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical