The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype - PubMed (original) (raw)
The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype
Jesmond Dalli et al. FASEB J. 2013 Jul.
Abstract
Maresins are produced by macrophages from docosahexaenoic acid (DHA) and exert potent proresolving and tissue homeostatic actions. Maresin 1 (MaR1; 7R,14S-dihydroxy-docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid) is the first identified maresin. Here, we investigate formation, stereochemistry, and precursor role of 13,14-epoxy-docosahexaenoic acid, an intermediate in MaR1 biosynthesis. The 14-lipoxygenation of DHA by human macrophage 12-lipoxygenase (hm12-LOX) gave 14-hydro(peroxy)-docosahexaenoic acid (14-HpDHA), as well as several dihydroxy-docosahexaenoic acids, implicating an epoxide intermediate formation by this enzyme. Using a stereo-controlled synthesis, enantiomerically pure 13S,14S-epoxy-docosa-4Z,7Z,9E,11E,16Z,19Z-hexaenoic acid (13S,14S-epoxy-DHA) was prepared, and its stereochemistry was confirmed by NMR spectroscopy. When this 13S,14S-epoxide was incubated with human macrophages, it was converted to MaR1. The synthetic 13S,14S-epoxide inhibited leukotriene B4 (LTB4) formation by human leukotriene A4 hydrolase (LTA4H) ∼40% (P<0.05) to a similar extent as LTA4 (∼50%, P<0.05) but was not converted to MaR1 by this enzyme. 13S,14S-epoxy-DHA also reduced (∼60%; P<0.05) arachidonic acid conversion by hm12-LOX and promoted conversion of M1 macrophages to M2 phenotype, which produced more MaR1 from the epoxide than M1. Together, these findings establish the biosynthesis of the 13S,14S-epoxide, its absolute stereochemistry, its precursor role in MaR1 biosynthesis, and its own intrinsic bioactivity. Given its actions and role in MaR1 biosynthesis, this epoxide is now termed 13,14-epoxy-maresin (13,14-eMaR) and exhibits new mechanisms in resolution of inflammation in its ability to inhibit proinflammatory mediator production by LTA4 hydrolase and to block arachidonate conversion by human 12-LOX rather than merely terminating phagocyte involvement.
Keywords: DHA; inflammation resolution; leukocytes; n-3 omega essential fatty acids; proresolving mediators.
Figures
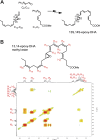
Figure 1.
Human macrophage LOX produces a novel 13_S_,14_S_-epoxide from DHA. A) Selected ion chromatogram (m/z 359-221) obtained from incubation of hm12-LOX (0.2 μM, 37°C, 10 min, pH 8) with DHA (5 μM). B) Incubations were stopped with ice-cold methanol, and products were extracted and assessed by metabololipidomics; MS-MS spectra were employed for the identification of compounds I–V. C) Acid alcohol trapping: hm12-LOX was incubated with DHA (20 s, 10 μM, 37°C) and stopped by addition of 10 vol of acidified (HCl) ice-cold methanol. MS-MS spectrum of the methoxy trapping products. Inset: selected ion chromatogram (m/z 373-297). Results are representative of n = 3 for each incubation condition.
Figure 2.
Total organic synthesis strategy for the production of 13_S_,14_S_-epoxy-DHA. A) Synthetic strategy and key precursors used for the preparation of 13_S_,14_S_-epoxy-DHA. B) Assignment of the Z or E stereochemistry for each C=C bond using 2-dimensional NMR spectroscopy. The 1H-1H gCOSY spectrum of a solution of the epoxide in C6D6 (_c_=9.6×10−3 M) shown was acquired on a Varian VNMRS 600 MHz NMR spectrometer at 25°C on a 5-mm triple resonance PFG 1H, 13C, 15N probe. The data were processed and analyzed on MestReNova 7.1.1 software and referenced to the C6D6 as an internal standard. This spectrum depicts all of the connectivities between adjacent alkenyl hydrogens (H4-H5, H7-H12, H16-H17, H19-H20). Colors denote a bitmap plotting method using a rainbow palette that gives depth to the positive and negative contours. The complete identification of each H atom using this method, in combination with its corresponding coupling constants (J values) enabled the unambiguous e/z assignment of all alkenyl hydrogens. The following chemical shifts and coupling constants were recorded: H9: 6.53 ppm, J = 11.2, 14.8 Hz; H11: 6.38 ppm, J = 11.2, 15.6 Hz; H10: 6.10 ppm, J = 10.8, 14.8 Hz; H8: 6.01, J = 11.2, 11.2 Hz; H4, H5, H7, H12, H16, H17, H19, H20: 5.55–5.25 ppm.
Figure 3.
Human macrophages convert synthetic 13_S_,14_S_-epoxy-DHA to MaR1. Human macrophages (1×107 cells) obtained from peripheral blood mononuclear cells were incubated with 13_S_,14_S_-epoxy-DHA (1.0 μM) intermediately prior to addition of STZ (0.1 mg; DPBS containing 10% BSA) and incubation for 60 min (37°C, pH 7.45). The incubations were stopped, and LM was assessed by metabololipidomics (see Materials and Methods). A) Selected ion chromatogram (m/z 359-141) depicting MaR1. B) MS-MS spectrum employed for identification of MaR1. C) Selected ion chromatogram (m/z 359-221) obtained following incubation of 13_S_,14_S_-epoxy-DHA with cells that were incubated at 100°C (60 min). Results are representative of n = 4 separate cell preparations.
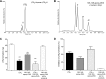
Figure 4.
13_S_,14_S_-epoxy-MaR inhibits LTB4 production by human recombinant LTA4H. Human LTA4H was incubated with LTA4 (10 μM), 13_S_,14_S_-epoxy-DHA (10 μM), or the aqueous hydrolysis products of 13_S_,14_S_-epoxy-DHA (10 μM; 30 min, 37°C, pH 8.0). The incubation was either stopped by addition of 2 vol ice-cold methanol or 10 μM of LTA4 was added, and the enzymes were incubated for 30 min (37°C, pH 8.0). The incubations were stopped, and LMs were obtained by solid-phase extraction (see Materials and Methods). A, B) Selected ion chromatograms for m/z 335-195 (A) and m/z 359-221 (B). Results are representative of n = 4 separate incubations. C, D) Quantification (C) and percentage inhibition (D) of LTB4 formation in the incubations by metabololipidomics. Values are expressed as means ±
se
; n = 4. **P < 0.01 vs. LTA4H + LTA4.
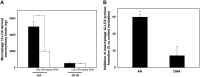
Figure 5.
13_S_,14_S_-epoxy-DHA selectively inhibits hm12-LOX AA lipoxygenation. 13_S_,14_S_-epoxy-DHA (2.5 μM) or vehicle (4% EtOH) was added to recombinant 12-LOX (1 μM) and incubated for 30 min (37°C, pH 8). AA or DHA (50 μM) was then added to the incubation mixture, the reaction was stopped after 5 min (room temperature), and products were assessed by metabololipidomics. Results are expressed as means ±
se
of 3 separate incubations. *P < 0.05 vs. corresponding control incubations.
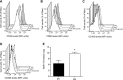
Figure 6.
13_S_,14_S_-epoxy DHA displays proresolving properties and is more readily converted by M2 macrophages. A–D) M1 macrophages were incubated with vehicle (Veh; PBS containing 0.1% EtOH), 13S,14S-epoxy-DHA, MaR1, or RvD1 (10 nM, pH 7.45, RPMI 0.1% FCS, 37°C) for 6 h. Expression of CD54 (A), CD80 (B), CD163 (C), and CD 206 (D) on the surface of these cells was assessed by flow cytometry using fluorescently conjugated antibodies. E) 13_S_,14_S_-epoxy-DHA (2 μM) was incubated with M1 or M2 macrophages (40×106 cells/ml, DPBS+/+, pH 7.45, 100 mg/ml BSA, 30 min, 37°C). Incubation was stopped with ice-cold methanol, and products were assessed by LM metabololipidomics. Results for A are means ±
se
; n = 3 separate cell preparations. Results for B–E are representative of n = 3 for each incubation condition.
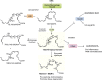
Figure 7.
Biosynthesis and actions of 13_S_,14_S_-epoxy-DHA and its role in MaR1 production. See text for details.
Similar articles
- Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages.
Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J, Serhan CN. Deng B, et al. PLoS One. 2014 Jul 18;9(7):e102362. doi: 10.1371/journal.pone.0102362. eCollection 2014. PLoS One. 2014. PMID: 25036362 Free PMC article. - Biosynthesis of the Maresin Intermediate, 13S,14S-Epoxy-DHA, by Human 15-Lipoxygenase and 12-Lipoxygenase and Its Regulation through Negative Allosteric Modulators.
Freedman C, Tran A, Tourdot BE, Kalyanaraman C, Perry S, Holinstat M, Jacobson MP, Holman TR. Freedman C, et al. Biochemistry. 2020 May 19;59(19):1832-1844. doi: 10.1021/acs.biochem.0c00233. Epub 2020 May 7. Biochemistry. 2020. PMID: 32324389 Free PMC article. - Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages.
Dalli J, Vlasakov I, Riley IR, Rodriguez AR, Spur BW, Petasis NA, Chiang N, Serhan CN. Dalli J, et al. Proc Natl Acad Sci U S A. 2016 Oct 25;113(43):12232-12237. doi: 10.1073/pnas.1607003113. Epub 2016 Oct 6. Proc Natl Acad Sci U S A. 2016. PMID: 27791009 Free PMC article. - Macrophage Proresolving Mediators-the When and Where.
Dalli J, Serhan C. Dalli J, et al. Microbiol Spectr. 2016 Jun;4(3):10.1128/microbiolspec.MCHD-0001-2014. doi: 10.1128/microbiolspec.MCHD-0001-2014. Microbiol Spectr. 2016. PMID: 27337457 Free PMC article. Review. - Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis.
Haeggström JZ. Haeggström JZ. J Biol Chem. 2004 Dec 3;279(49):50639-42. doi: 10.1074/jbc.R400027200. Epub 2004 Aug 31. J Biol Chem. 2004. PMID: 15339917 Review. No abstract available.
Cited by
- The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution.
Serhan CN, Chiang N, Dalli J. Serhan CN, et al. Semin Immunol. 2015 May;27(3):200-15. doi: 10.1016/j.smim.2015.03.004. Epub 2015 Apr 7. Semin Immunol. 2015. PMID: 25857211 Free PMC article. Review. - The Immune System in Tissue Environments Regaining Homeostasis after Injury: Is "Inflammation" Always Inflammation?
Kulkarni OP, Lichtnekert J, Anders HJ, Mulay SR. Kulkarni OP, et al. Mediators Inflamm. 2016;2016:2856213. doi: 10.1155/2016/2856213. Epub 2016 Aug 11. Mediators Inflamm. 2016. PMID: 27597803 Free PMC article. Review. - Regulation of Apoptotic Cell Clearance During Resolution of Inflammation.
Arienti S, Barth ND, Dorward DA, Rossi AG, Dransfield I. Arienti S, et al. Front Pharmacol. 2019 Aug 13;10:891. doi: 10.3389/fphar.2019.00891. eCollection 2019. Front Pharmacol. 2019. PMID: 31456686 Free PMC article. Review. - Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation.
Sugimoto S, Mena HA, Sansbury BE, Kobayashi S, Tsuji T, Wang CH, Yin X, Huang TL, Kusuyama J, Kodani SD, Darcy J, Profeta G, Pereira N, Tanzi RE, Zhang C, Serwold T, Kokkotou E, Goodyear LJ, Cypess AM, Leiria LO, Spite M, Tseng YH. Sugimoto S, et al. Nat Metab. 2022 Jun;4(6):775-790. doi: 10.1038/s42255-022-00590-0. Epub 2022 Jun 27. Nat Metab. 2022. PMID: 35760872 Free PMC article. - Maresin-1 Prevents Liver Fibrosis by Targeting Nrf2 and NF-κB, Reducing Oxidative Stress and Inflammation.
Rodríguez MJ, Sabaj M, Tolosa G, Herrera Vielma F, Zúñiga MJ, González DR, Zúñiga-Hernández J. Rodríguez MJ, et al. Cells. 2021 Dec 3;10(12):3406. doi: 10.3390/cells10123406. Cells. 2021. PMID: 34943914 Free PMC article.
References
- Weissmann G. (1978) Leukocytes as secretory organs of inflammation. Hosp. Pract. 13, 53–62 - PubMed
- Shimizu T. (2009) Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 49, 123–150 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous