Microglia, seen from the CX3CR1 angle - PubMed (original) (raw)
Microglia, seen from the CX3CR1 angle
Yochai Wolf et al. Front Cell Neurosci. 2013.
Abstract
Microglial cells in brain and spinal cord are characterized by high expression of the chemokine receptor CX3CR1. Expression of the sole CX3CR1 ligand, the membrane-tethered and sheddable chemokine CX3CL1/fractalkine, is restricted in the brain parenchyma to selected neurons. Here we summarize our current understanding of the physiological role of CX3CR1 for microglia function and the CX3C axis in microglial/neuronal crosstalk in homeostasis and under challenge. Moreover, we will discuss the efforts of our laboratory and others to exploit CX3CR1 promoter activity for the visualization and genetic manipulation of microglia to probe their functional contributions in the central nerve system (CNS) context.
Keywords: CX3CR1; Cre-loxP knock-in mice; microglia; neuroimmunology; neuropathology.
Figures
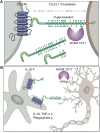
Figure 1
(A) Schematic of CX3C chemokine family and (B) potential scenario of differential outcomes of neuronal shed and membrane-anchored CX3CL1 engagement by microglia inducing or suppressing microglial IL-1β production, respectively.
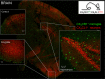
Figure 2
Brain section of CX3CR1gfp:CX3CL1cherry double reporter animals (Kim et al., 2011) highlighting CX3CR1-expressing microglia and subsets of CX3CL1-expressing neurons in specific brain regions. Note that both reporters are expressed in the cytoplasm and not as fusion proteins. Thus they reflect the respective promoter activity but not the presence of the respective proteins. Hence no co-localization has to be expected.
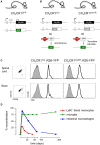
Figure 3
(A) Schematic of modified CX3CR1 loci of CX3CR1gfp mice (Jung et al., 2000) and (B) CX3CR1Cre and CX3CR1CreER mice (Yona et al., 2013); (C) Efficient YFP labeling of both spinal cord and brain microglia in CX3CR1CreER and CX3CR1Cre mice crossed with R26-YFP reporter mice. CX3CR1CreER:R26-YFP mice were treated with tamoxifen to induce the rearrangement (Yona et al., 2013); (D) Induction but subsequent progressive loss of cells harboring gene rearrangements in peripheral myeloid cells (monocytes, intestinal macrophages) and persistence of genomic modification in microglial cells.
Similar articles
- Fractalkine/CX3CR1 is involved in the cross-talk between neuron and glia in neurological diseases.
Luo P, Chu SF, Zhang Z, Xia CY, Chen NH. Luo P, et al. Brain Res Bull. 2019 Mar;146:12-21. doi: 10.1016/j.brainresbull.2018.11.017. Epub 2018 Nov 26. Brain Res Bull. 2019. PMID: 30496784 Review. - Analysis of the Role of CX3CL1 (Fractalkine) and Its Receptor CX3CR1 in Traumatic Brain and Spinal Cord Injury: Insight into Recent Advances in Actions of Neurochemokine Agents.
Poniatowski ŁA, Wojdasiewicz P, Krawczyk M, Szukiewicz D, Gasik R, Kubaszewski Ł, Kurkowska-Jastrzębska I. Poniatowski ŁA, et al. Mol Neurobiol. 2017 Apr;54(3):2167-2188. doi: 10.1007/s12035-016-9787-4. Epub 2016 Mar 1. Mol Neurobiol. 2017. PMID: 26927660 Free PMC article. Review. - Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS.
Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Hughes PM, et al. Glia. 2002 Mar 15;37(4):314-27. Glia. 2002. PMID: 11870871 - Fractalkine/CX3CR1-Dependent Modulation of Synaptic and Network Plasticity in Health and Disease.
Camacho-Hernández NP, Peña-Ortega F. Camacho-Hernández NP, et al. Neural Plast. 2023 Jan 4;2023:4637073. doi: 10.1155/2023/4637073. eCollection 2023. Neural Plast. 2023. PMID: 36644710 Free PMC article. Review. - Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine.
Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Zhuang ZY, et al. Brain Behav Immun. 2007 Jul;21(5):642-51. doi: 10.1016/j.bbi.2006.11.003. Epub 2006 Dec 15. Brain Behav Immun. 2007. PMID: 17174525 Free PMC article.
Cited by
- Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety.
Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Reader BF, et al. Neuroscience. 2015 Mar 19;289:429-42. doi: 10.1016/j.neuroscience.2015.01.001. Epub 2015 Jan 14. Neuroscience. 2015. PMID: 25596319 Free PMC article. Review. - Fractalkine Receptor Deficiency Is Associated with Early Protection but Late Worsening of Outcome following Brain Trauma in Mice.
Zanier ER, Marchesi F, Ortolano F, Perego C, Arabian M, Zoerle T, Sammali E, Pischiutta F, De Simoni MG. Zanier ER, et al. J Neurotrauma. 2016 Jun 1;33(11):1060-72. doi: 10.1089/neu.2015.4041. Epub 2015 Sep 8. J Neurotrauma. 2016. PMID: 26180940 Free PMC article. - Astrocytes and microglia: Models and tools.
Guttenplan KA, Liddelow SA. Guttenplan KA, et al. J Exp Med. 2019 Jan 7;216(1):71-83. doi: 10.1084/jem.20180200. Epub 2018 Dec 12. J Exp Med. 2019. PMID: 30541903 Free PMC article. Review. - Neuronal apoptosis drives remodeling states of microglia and shifts in survival pathway dependence.
Anderson SR, Roberts JM, Ghena N, Irvin EA, Schwakopf J, Cooperstein IB, Bosco A, Vetter ML. Anderson SR, et al. Elife. 2022 Apr 28;11:e76564. doi: 10.7554/eLife.76564. Elife. 2022. PMID: 35481836 Free PMC article. - Microglia lacking a peroxisomal β-oxidation enzyme chronically alter their inflammatory profile without evoking neuronal and behavioral deficits.
Beckers L, Geric I, Stroobants S, Beel S, Van Damme P, D'Hooge R, Baes M. Beckers L, et al. J Neuroinflammation. 2019 Mar 13;16(1):61. doi: 10.1186/s12974-019-1442-3. J Neuroinflammation. 2019. PMID: 30866963 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous