Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144 - PubMed (original) (raw)
. 2013 Jun 7;112(12):1592-601.
doi: 10.1161/CIRCRESAHA.112.300626. Epub 2013 Mar 21.
Noemi Rotllan, Alexander V Vlassov, Alberto Dávalos, Mu Li, Leigh Goedeke, Juan F Aranda, Daniel Cirera-Salinas, Elisa Araldi, Alessandro Salerno, Amarylis Wanschel, Jiri Zavadil, Antonio Castrillo, Jungsu Kim, Yajaira Suárez, Carlos Fernández-Hernando
Affiliations
- PMID: 23519695
- PMCID: PMC3929583
- DOI: 10.1161/CIRCRESAHA.112.300626
Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144
Cristina M Ramírez et al. Circ Res. 2013.
Abstract
Rationale: Foam cell formation because of excessive accumulation of cholesterol by macrophages is a pathological hallmark of atherosclerosis, the major cause of morbidity and mortality in Western societies. Liver X nuclear receptors (LXRs) regulate the expression of the adenosine triphosphate-binding cassette (ABC) transporters, including adenosine triphosphate-binding cassette transporter A1 (ABCA1) and adenosine triphosphate-binding cassette transporter G1 (ABCG1). ABCA1 and ABCG1 facilitate the efflux of cholesterol from macrophages and regulate high-density lipoprotein (HDL) biogenesis. Increasing evidence supports the role of microRNA (miRNAs) in regulating cholesterol metabolism through ABC transporters.
Objective: We aimed to identify novel miRNAs that regulate cholesterol metabolism in macrophages stimulated with LXR agonists.
Methods and results: To map the miRNA expression signature of macrophages stimulated with LXR agonists, we performed an miRNA profiling microarray analysis in primary mouse peritoneal macrophages stimulated with LXR ligands. We report that LXR ligands increase miR-144 expression in macrophages and mouse livers. Overexpression of miR-144 reduces ABCA1 expression and attenuates cholesterol efflux to apolipoproteinA1 in macrophages. Delivery of miR-144 oligonucleotides to mice attenuates ABCA1 expression in the liver, reducing HDL levels. Conversely, silencing of miR-144 in mice increases the expression of ABCA1 and plasma HDL levels. Thus, miR-144 seems to regulate both macrophage cholesterol efflux and HDL biogenesis in the liver.
Conclusions: miR-144 regulates cholesterol metabolism via suppressing ABCA1 expression and modulation of miRNAs may represent a potential therapeutical intervention for treating dyslipidemia and atherosclerotic vascular disease.
Keywords: ABCA1; cardiovascular research; cholesterol efflux; cholesterol homeostatis; high-density lipoprotein; lipids and lipoprotein metabolism; microRNAs.
Figures
Figure 1. miR-144 expression is up-regulated in LXR-treated macrophages and has multiple lipid-related predicted target genes
(A) miRNA array analysis of miRNAs up-regulated (red) or down-regulated (green) from peritoneal macrophages treated with T090 (n=4). (B to D) Gene ontology analysis of the miR-144 predicted target genes using Panther software. (E to F) Protein-protein interaction analysis of the miR-144 predicted targets using String software and Navigator 2.2.
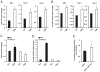
Figure 2. LXR induces miR-144 transcriptional expression
(A) qRT-PCR analysis of miR-144 expression in mouse peritoneal macrophages (MØ, left panel), THP-1 cells (middle panel) and Huh-7 cells (right panel) treated with T090. (B) qRT-PCR analysis of pri-miR-144/451 expression in mouse peritoneal macrophages (left panel), THP-1 cells (middle panel) and Huh-7 cells (right panel) treated with T090. (C and D) qRT-PCR analysis of miR-144 (C) and pri-miR-144/451 (D) expression from wild-type (WT) and LXRα,β-/- macrophages treated with T090. (E) miR-144/451 promoter-luciferase reporter activity in Huh-7 cells treated with T090. Data are expressed as relative expression and correspond to mean±SEM of 3 independent experiments. *Significantly different from cells without treatment (Ctrl), _P_≤0.05.
Figure 3. miR-144 levels regulate ABCA1 expression
(A) qRT-PCR analysis of ABCA1, and ABCG1 expression in mouse peritoneal macrophages transfected with miR-144 mimics. Data are expressed as relative expression and correspond to the mean±SEM from 3 independent experiments. *Significantly different from cells transfected with control mimic (Con-miR), *P ≤ 0.05. (B and C) Western blot analysis of ABCA1, ABCG1 and HSP90 in mouse peritoneal macrophages and THP-1 cells transfected with (B) Con-miR or miR-144, or (C) control inhibitor (Con-inh) or anti-miR-144 in the presence or absence of Ac-LDL (Ac) or T090. Data correspond to a representative experiment among three that gave similar results. Values of the band densitometry analysis are shown. (D) Luciferase-reporter activity in COS-7 cells transfected with Con-miR or miR-144 (40 nM) mimic and human Abca1 3’UTR containing the indicated point mutations (PM) in the miR-144 target sites. Data are expressed as mean % of 3’UTR activity of Con-miR ±SEM and are representative of ≥3 experiments. *Significantly different from cells cotransfected with Con-miR and wild-type (WT) 3’UTR. _P_≤ 0.05. (E) Human Abca1 3’UTR containing the indicated point mutations (PM) in the miR-144 target sites.
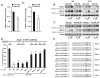
Figure 4. miR-33 and miR-144 cooperate to regulate ABCA1 expression
(A) Western blot analysis of Huh-7 cells transfected with 5nM non-targeting control mimic (Con-miR), miR-33, miR-144 and miR-33/miR-144 (upper panel). Data correspond to a representative experiment among three that gave similar results. Quantification of specific bands was conducted by densitometric analysis and is shown as the bottom of each band. (B) Activity of the luciferase reporter construct fused to the 3’UTR of Abca1 in COS-7 cells. Cells were transfected with Con-miR or miR-33, or miR-144, or miR-33 and miR-144 together. All microRNA constructs were transfected at 5νM final concentration. Relative luciferase activity is presented and data are the mean±SEM of 3 independent experiments in triplicate. *Significantly different from cells cotransfected with Con-miR and Abca1 3’UTR. _P_≤ 0.05. # Significantly different from cells cotransfected with miR-33 or miR-144 and Abca1 3’UTR. _P_≤ 0.05. (C) Total cholesterol efflux to ApoA1 in Huh-7 cells stimulated with T090 and expressing control miR (Con-miR), miR-33, miR-144 or miR-33 and miR-144. Data are the mean±SEM of 2 independent experiments performed in triplicate.
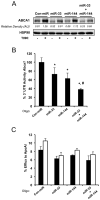
Figure 5. Modulation of miR-144 regulates macrophage cholesterol efflux
(A) Total cholesterol efflux to ApoA1 in J774 mouse macrophages stimulated with Ac-LDL (Ac) or T090 and expressing control miR (Con-miR) or miR-144. Upper panel shows the ABCA1 protein expression assessed by western blotting in the same conditions. (B) Total cholesterol efflux to ApoA1 in J774 mouse macrophages stimulated with Ac-LDL (Ac) or T090 and expressing control inhibitor (Con-inh) or Inh-miR-144. Upper panel shows ABCA1 protein expression assessed by western blotting in the same conditions. Data are the mean±SEM of 3 independent experiments performed in triplicate. *Significantly different from cells transfected with Con-miR (A) or Con-inh (B). _P_≤ 0.05.
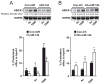
Figure 6. Delivery of miR-144 oligonucleotides significantly reduces liver ABCA1 expression and HDL levels in mice
(A) qRT-PCR analysis of ABCA1 and ABCG1 expression from mouse livers treated with miR-144 mimics. Data are the mean±SEM from 6 mice per group. *Significantly different from mice injected with non-targeting control mimic (Con-miR) conjugated particles. _P_≤ 0.05. (B) Analysis of hepatic gene expression 6 days after injection of Con-miR or miR-144. Western blot analysis of liver tissue from three representative mice per group. Quantification analysis is shown in the right panel. Data are the mean±SEM from 6 mice per group. *Significantly different from mice injected with Con-miR conjugated particles. _P_≤ 0.05. (C) Total cholesterol (left panel), HDL cholesterol (middle panel) and TG (right panel) levels in mice treated with Con-miR or miR-144 mimics (n=6). *Significantly different from mice injected with non-targeting Con-miR conjugated particles. _P_≤ 0.05. (D) Lipoprotein profile from mice treated with Con-miR or miR-144 mimics (n=6). Cholesterol distribution across plasma fractions was analyzed by FPLC.
Figure 7. Inhibition of miR-144 increases hepatic ABCA1 expression and raises HDL plasma levels
(A) qRT-PCR analysis of miR-144 levels from mouse livers treated with miR-144 inhibitor (Inh-miR-144) conjugated particles. Data are expressed as relative expression and correspond to the mean±SEM from 6 mice per group. *Significantly different from mice injected with control inhibitor (Con-inh), #significantly different from mice untreated and injected with Con-Inh or treated with T090 and injected with Inh-miR-144 compared with mice treated with T090 and injected with Con-Inh, _P_≤0.05. (B) qRT-PCR analysis of hepatic ABCA1 gene expression mouse liver treated or untreated with T090 and control inhibitor (Con-inh) or Inh-miR-144. Significantly different from mice injected with Con-inh, _P_≤0.05. (C) Western blot analysis of ABCA1 expression from liver tissue of mice injected with Con-inh or Inh-miR-144 and treated with vehicle (upper panel) or T090 (bottom panel). Quantification of specific bands was conducted by densitometric analysis and is shown at the bottom of each band. (D) Total cholesterol, HDL cholesterol and TG plasma levels in mice injected with Con-miR or miR-144 mimics and treated or not with T090 (n=6). Data are the mean ± SEM from 6 mice per group. Significantly different from mice injected with Con-inh, *P ≤ 0.05. (E) Lipoprotein profile analysis from mice treated with Con-inh or Inh-miR-144 (n=6). Cholesterol distribution across plasma fractions was analyzed by FPLC.
Comment in
- Nuclear receptors and microRNA-144 coordinately regulate cholesterol efflux.
Vickers KC, Rader DJ. Vickers KC, et al. Circ Res. 2013 Jun 7;112(12):1529-31. doi: 10.1161/CIRCRESAHA.113.301422. Circ Res. 2013. PMID: 23743223 Free PMC article.
Similar articles
- Activation of liver X receptor decreases atherosclerosis in Ldlr⁻/⁻ mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells.
Kappus MS, Murphy AJ, Abramowicz S, Ntonga V, Welch CL, Tall AR, Westerterp M. Kappus MS, et al. Arterioscler Thromb Vasc Biol. 2014 Feb;34(2):279-84. doi: 10.1161/ATVBAHA.113.302781. Epub 2013 Dec 5. Arterioscler Thromb Vasc Biol. 2014. PMID: 24311381 Free PMC article. - MicroRNA-144 regulates hepatic ATP binding cassette transporter A1 and plasma high-density lipoprotein after activation of the nuclear receptor farnesoid X receptor.
de Aguiar Vallim TQ, Tarling EJ, Kim T, Civelek M, Baldán Á, Esau C, Edwards PA. de Aguiar Vallim TQ, et al. Circ Res. 2013 Jun 7;112(12):1602-12. doi: 10.1161/CIRCRESAHA.112.300648. Epub 2013 Mar 21. Circ Res. 2013. PMID: 23519696 Free PMC article. - Group X secretory phospholipase A2 negatively regulates ABCA1 and ABCG1 expression and cholesterol efflux in macrophages.
Shridas P, Bailey WM, Gizard F, Oslund RC, Gelb MH, Bruemmer D, Webb NR. Shridas P, et al. Arterioscler Thromb Vasc Biol. 2010 Oct;30(10):2014-21. doi: 10.1161/ATVBAHA.110.210237. Arterioscler Thromb Vasc Biol. 2010. PMID: 20844270 Free PMC article. - ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: important targets for the treatment of atherosclerosis.
Ye D, Lammers B, Zhao Y, Meurs I, Van Berkel TJ, Van Eck M. Ye D, et al. Curr Drug Targets. 2011 May;12(5):647-60. doi: 10.2174/138945011795378522. Curr Drug Targets. 2011. PMID: 21039336 Review. - HDL, cholesterol efflux, and ABCA1: Free from good and evil dualism.
Ogura M. Ogura M. J Pharmacol Sci. 2022 Oct;150(2):81-89. doi: 10.1016/j.jphs.2022.07.004. Epub 2022 Aug 6. J Pharmacol Sci. 2022. PMID: 36055755 Review.
Cited by
- Cellular and Molecular Mechanisms Underlying Glioblastoma and Zebrafish Models for the Discovery of New Treatments.
Reimunde P, Pensado-López A, Carreira Crende M, Lombao Iglesias V, Sánchez L, Torrecilla-Parra M, Ramírez CM, Anfray C, Torres Andón F. Reimunde P, et al. Cancers (Basel). 2021 Mar 3;13(5):1087. doi: 10.3390/cancers13051087. Cancers (Basel). 2021. PMID: 33802571 Free PMC article. Review. - Role of miRNA in the Regulatory Mechanisms of Estrogens in Cardiovascular Ageing.
Pérez-Cremades D, Mompeón A, Vidal-Gómez X, Hermenegildo C, Novella S. Pérez-Cremades D, et al. Oxid Med Cell Longev. 2018 Dec 20;2018:6082387. doi: 10.1155/2018/6082387. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30671171 Free PMC article. Review. - miR-206 controls LXRα expression and promotes LXR-mediated cholesterol efflux in macrophages.
Vinod M, Chennamsetty I, Colin S, Belloy L, De Paoli F, Schaider H, Graier WF, Frank S, Kratky D, Staels B, Chinetti-Gbaguidi G, Kostner GM. Vinod M, et al. Biochim Biophys Acta. 2014 Jun;1841(6):827-35. doi: 10.1016/j.bbalip.2014.02.006. Epub 2014 Mar 3. Biochim Biophys Acta. 2014. PMID: 24603323 Free PMC article. - Modulation of Hypercholesterolemia-Induced Oxidative/Nitrative Stress in the Heart.
Csonka C, Sárközy M, Pipicz M, Dux L, Csont T. Csonka C, et al. Oxid Med Cell Longev. 2016;2016:3863726. doi: 10.1155/2016/3863726. Epub 2015 Dec 14. Oxid Med Cell Longev. 2016. PMID: 26788247 Free PMC article. Review. - MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels.
Goedeke L, Rotllan N, Canfrán-Duque A, Aranda JF, Ramírez CM, Araldi E, Lin CS, Anderson NN, Wagschal A, de Cabo R, Horton JD, Lasunción MA, Näär AM, Suárez Y, Fernández-Hernando C. Goedeke L, et al. Nat Med. 2015 Nov;21(11):1280-9. doi: 10.1038/nm.3949. Epub 2015 Oct 5. Nat Med. 2015. PMID: 26437365 Free PMC article.
References
- Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. - PubMed
- Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. Abca1 and abcg1 synergize to mediate cholesterol export to apoa-i. Arterioscler Thromb Vasc Biol. 2006;26:534–540. - PubMed
- Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. - PubMed
- Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. Hdl, abc transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL105945/HL/NHLBI NIH HHS/United States
- R01 HL107953/HL/NHLBI NIH HHS/United States
- R01HL107953/HL/NHLBI NIH HHS/United States
- R01 HL106063/HL/NHLBI NIH HHS/United States
- P30NS069329/NS/NINDS NIH HHS/United States
- P30 NS069329/NS/NINDS NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- R01 HL105945/HL/NHLBI NIH HHS/United States
- R01HL106063/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous