Temsirolimus combined with sorafenib in hepatocellular carcinoma: a phase I dose-finding trial with pharmacokinetic and biomarker correlates - PubMed (original) (raw)
Clinical Trial
. 2013 Jul;24(7):1900-1907.
doi: 10.1093/annonc/mdt109. Epub 2013 Mar 21.
H S Nimeiri 2, P N Munster 3, M T Vergo 2, Y Huang 4, C-M Li 4, J Hwang 3, M F Mulcahy 2, B M Yeh 5, P Kuhn 6, M S Luttgen 6, J A Grabowsky 3, L Stucky-Marshall 2, W M Korn 3, A H Ko 3, E K Bergsland 3, A B Benson 3rd 2, A P Venook 3
Affiliations
- PMID: 23519998
- PMCID: PMC3690907
- DOI: 10.1093/annonc/mdt109
Clinical Trial
Temsirolimus combined with sorafenib in hepatocellular carcinoma: a phase I dose-finding trial with pharmacokinetic and biomarker correlates
R K Kelley et al. Ann Oncol. 2013 Jul.
Abstract
Background: Based upon preclinical evidence for improved antitumor activity in combination, this phase I study investigated the maximum-tolerated dose (MTD), safety, activity, pharmacokinetics (PK), and biomarkers of the mammalian target of rapamycin inhibitor, temsirolimus, combined with sorafenib in hepatocellular carcinoma (HCC).
Patients and methods: Patients with incurable HCC and Child Pugh score ≤B7 were treated with sorafenib plus temsirolimus by 3 + 3 design. The dose-limiting toxicity (DLT) interval was 28 days. The response was assessed every two cycles. PK of temsirolimus was measured in a cohort at MTD.
Results: Twenty-five patients were enrolled. The MTD was temsirolimus 10 mg weekly plus sorafenib 200 mg twice daily. Among 18 patients at MTD, DLT included grade 3 hand-foot skin reaction (HFSR) and grade 3 thrombocytopenia. Grade 3 or 4 related adverse events at MTD included hypophosphatemia (33%), infection (22%), thrombocytopenia (17%), HFSR (11%), and fatigue (11%). With sorafenib, temsirolimus clearance was more rapid (P < 0.05). Two patients (8%) had a confirmed partial response (PR); 15 (60%) had stable disease (SD). Alpha-fetoprotein (AFP) declined ≥50% in 60% assessable patients.
Conclusion: The MTD of sorafenib plus temsirolimus in HCC was lower than in other tumor types. HCC-specific phase I studies are necessary. The observed efficacy warrants further study.
Keywords: hepatocellular carcinoma; mTOR; pharmacokinetics; sorafenib; temsirolimus.
Figures
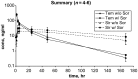
Figure 1.
Sorafenib effect on temsirolimus and sirolimus concentration. Temsirolimus AUCinf was lower and clearance more rapid in the presence of sorafenib by a statistical _t_-test (P < 0.05) though interpretation is limited by small sample size (n = 4–6). The 72 and 96 h time points were optional and not obtained in any patient.
References
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi:10.1056/NEJMoa0708857. - DOI - PubMed
- Cheng A, Kang Y, Lin D, Park J, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC). In American Society of Clinical Oncology Annual Meeting. Chicago, IL. J Clin Oncol. 2011 29(15-suppl): 4000.
- Llovet JM, Decaen T, Raoul J-L, Boucher E, et al. Brivanib versus placebo in patients with advanced hepatocellular carcinoma (HCC) who failed or were intolerant to sorafenib: results from the phase 3 BRISK-PS study. International Liver Congress, European Association for the Study of the Liver; Barcelona, Spain. 2012.
- Zhu RO, Evans J, Ross P, et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with hepatocellular carcinoma (HCC). 37th ESMO Congress, European Society of Medical Oncology; Vienna, Austria. 2012. - PubMed
- Menon S, Yecies JL, Zhang HH, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5:ra24. doi:10.1126/scisignal.2002739. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous