The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance - PubMed (original) (raw)
Review
The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance
Ellen L Burnham et al. Eur Respir J. 2014 Jan.
Abstract
Acute respiratory distress syndrome (ARDS) continues to be a major healthcare problem, affecting >190,000 people in the USA annually, with a mortality of 27-45%, depending on the severity of the illness and comorbidities. Despite advances in clinical care, particularly lung protective strategies of mechanical ventilation, most survivors experience impaired health-related quality of life for years after the acute illness. While most patients survive the acute illness, a subset of ARDS survivors develops a fibroproliferative response characterised by fibroblast accumulation and deposition of collagen and other extracellular matrix components in the lung. Historically, the development of severe fibroproliferative lung disease has been associated with a poor prognosis with high mortality and/or prolonged ventilator dependence. More recent studies also support a relationship between the magnitude of the fibroproliferative response and long-term health-related quality of life. The factors that determine which patients develop fibroproliferative ARDS and the cellular mechanisms responsible for this pathological response are not well understood. This article reviews our current understanding of the contribution of pulmonary dysfunction to mortality and to quality of life in survivors of ARDS, the mechanisms driving pathological fibroproliferation and potential therapeutic approaches to prevent or attenuate fibroproliferative lung disease.
Conflict of interest statement
Conflict of interest: None declared.
Figures
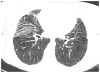
FIGURE 1
High-resolution computed tomography image of the base of the lungs of an actue respiratory distress syndrome survivor demonstrating persistent reticular changes in the lungs and traction bronchiectasis 18 months after the acute illness.
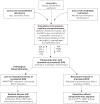
FIGURE 2
Mechanisms driving fibroproliferation in acute respiratory distress syndrome. Characteristic alterations related to lung injury evolve over time, and may be influenced by a variety of environmental and patient-specific factors. In response to alveolar–capillary injury, a provisional extracellular matrix (ECM) is ultimately formed to promote repair, and proteinaceous pulmonary oedema fluid is cleared from the alveolar space. The provisional ECM largely resolves after restoration of lung architecture; however, a subset of patients will not clear the provisional ECM and exhibit evidence of exuberant fibroproliferation with fibrosis lasting from months to years after the acute event. Bold type represents major “activity” at this time point. AT: angiotensin; TGF: transforming growth factor; IGF: insulin-like growth factor; PDGF: platelet-derived growth factor; FGF: fibroblast growth factor; KGF: keratinocyte growth factor; PGE: prostaglandin E; HGF: hepatocyte growth factor; MMP: matrix metalloproteinases; TIMP: tissue inhibitor of metalloproteinase; PFT: pulmonary function test; HRCT: high-resolution computed tomography.
Similar articles
- PTPα promotes fibroproliferative responses after acute lung injury.
Aschner Y, Correll KA, Beke KM, Foster DG, Roybal HM, Nelson MR, Meador CL, Strand M, Anderson KC, Moore CM, Reynolds PR, Kopf KW, Burnham EL, Downey GP. Aschner Y, et al. Am J Physiol Lung Cell Mol Physiol. 2022 Jul 1;323(1):L69-L83. doi: 10.1152/ajplung.00436.2021. Epub 2022 Jun 7. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 35670474 Free PMC article. - The alveolar fibroproliferative response in moderate to severe COVID-19-related acute respiratory distress syndrome and 1-yr follow-up.
Zhang S, Boers LS, de Brabander J, van den Heuvel LB, Blok SG, Kullberg RFJ, Smids-Dierdorp BS, Dekker T, Aberson HL, Meijboom LJ, Vlaar APJ, Heunks L, Nossent EJ, van der Poll T, Bos LDJ, Duitman J; ArtDECO consortium and the Amsterdam UMC COVID study group. Zhang S, et al. Am J Physiol Lung Cell Mol Physiol. 2024 Jan 1;326(1):L7-L18. doi: 10.1152/ajplung.00156.2023. Epub 2023 Nov 7. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 37933449 - Advances in mechanisms of repair and remodelling in acute lung injury.
Dos Santos CC. Dos Santos CC. Intensive Care Med. 2008 Apr;34(4):619-30. doi: 10.1007/s00134-007-0963-x. Epub 2008 Feb 9. Intensive Care Med. 2008. PMID: 18264692 Review. - Pulmonary redox imbalance drives early fibroproliferative response in moderate/severe coronavirus disease-19 acute respiratory distress syndrome and impacts long-term lung abnormalities.
Yang C, Tan Y, Li Z, Hu L, Chen Y, Zhu S, Hu J, Huai T, Li M, Zhang G, Rao D, Fei G, Shao M, Ding Z. Yang C, et al. Ann Intensive Care. 2024 May 12;14(1):72. doi: 10.1186/s13613-024-01293-3. Ann Intensive Care. 2024. PMID: 38735020 Free PMC article. - Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome.
Cabrera-Benitez NE, Laffey JG, Parotto M, Spieth PM, Villar J, Zhang H, Slutsky AS. Cabrera-Benitez NE, et al. Anesthesiology. 2014 Jul;121(1):189-98. doi: 10.1097/ALN.0000000000000264. Anesthesiology. 2014. PMID: 24732023 Free PMC article. Review.
Cited by
- Pharmacological targets and validation of remdesivir for the treatment of COVID-19-associated pulmonary fibrosis: A network-based pharmacology and bioinformatics study.
Zhao X, Yang L. Zhao X, et al. Medicine (Baltimore). 2024 Sep 27;103(39):e39062. doi: 10.1097/MD.0000000000039062. Medicine (Baltimore). 2024. PMID: 39331891 Free PMC article. - Diagnostic performance of radiologists in distinguishing post-COVID-19 residual abnormalities from interstitial lung abnormalities.
Lee JE, Lee HJ, Park G, Chae KJ, Jin KN, Castañer E, Ghaye B, Ko JP, Prosch H, Simpson S, Larici AR, Kanne JP, Frauenfelder T, Jeong YJ, Yoon SH. Lee JE, et al. Eur Radiol. 2024 Sep 23. doi: 10.1007/s00330-024-11075-x. Online ahead of print. Eur Radiol. 2024. PMID: 39311916 - TGF-β1 overexpression in severe COVID-19 survivors and its implications for early-phase fibrotic abnormalities and long-term functional impairment.
Alfaro E, Casitas R, Díaz-García E, García-Tovar S, Galera R, Torres-Vargas M, Fernández-Velilla M, López-Fernández C, Añón JM, Quintana-Díaz M, García-Río F, Cubillos-Zapata C. Alfaro E, et al. Front Immunol. 2024 Aug 29;15:1401015. doi: 10.3389/fimmu.2024.1401015. eCollection 2024. Front Immunol. 2024. PMID: 39281687 Free PMC article. - Deficiency and dysfunctional roles of natural killer T cells in patients with ARDS.
Park KJ, Kim TO, Cho YN, Jin HM, Jo YG, Shin HJ, Kho BG, Kee SJ, Park YW. Park KJ, et al. Front Immunol. 2024 Aug 30;15:1433028. doi: 10.3389/fimmu.2024.1433028. eCollection 2024. Front Immunol. 2024. PMID: 39281681 Free PMC article. - Diazepam inhibits LPS-induced pyroptosis and inflammation and alleviates pulmonary fibrosis in mice by regulating the let-7a-5p/MYD88 axis.
Song D, Tang X, Du J, Tao K, Li Y. Song D, et al. PLoS One. 2024 Jun 14;19(6):e0305409. doi: 10.1371/journal.pone.0305409. eCollection 2024. PLoS One. 2024. PMID: 38875245 Free PMC article.
References
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2:319–323. - PubMed
- Ranieri VM, Rubenfeld GD, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. - PubMed
- Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. - PubMed
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL109517/HL/NHLBI NIH HHS/United States
- 1R01HL109517-01A1/HL/NHLBI NIH HHS/United States
- R01 NR011051/NR/NINR NIH HHS/United States
- R01 HL090669/HL/NHLBI NIH HHS/United States
- HL090669/HL/NHLBI NIH HHS/United States
- R24 AA019661/AA/NIAAA NIH HHS/United States
- K24 HL089223/HL/NHLBI NIH HHS/United States
- P50 HL074024/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical