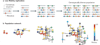The role of mutational robustness in RNA virus evolution - PubMed (original) (raw)
Review
The role of mutational robustness in RNA virus evolution
Adam S Lauring et al. Nat Rev Microbiol. 2013 May.
Abstract
RNA viruses face dynamic environments and are masters at adaptation. During their short 'lifespans', they must surmount multiple physical, anatomical and immunological challenges. Central to their adaptative capacity is the enormous genetic diversity that characterizes RNA virus populations. Although genetic diversity increases the rate of adaptive evolution, low replication fidelity can present a risk because excess mutations can lead to population extinction. In this Review, we discuss the strategies used by RNA viruses to deal with the increased mutational load and consider how this mutational robustness might influence viral evolution and pathogenesis.
Figures
Figure 1. Viral populations as mutant networks
a| The consensus sequence (grey line) is the average sequence of a population and might not be represented on any individual genome because of the extremely high genetic diversity of RNA virus populations. Low-fidelity replication, which is a characteristic feature of RNA viruses, results in a diverse population of unique genotypic variants while maintaining the same consensus genome sequence. Mutations acquired in each replication cycle are represented by differently coloured triangles. b| RNA virus populations can be depicted as networks in which the genetic variants (circles) of varying fitness are connected by mutational pathways (black lines).
Figure 2. High mutation rates and survival of the flattest
In a fitness landscape, the ‘ground level’ is a two-dimensional representation of genotypic sequence space, and the vertical axis gives the fitness value for each genotype or sequence.a| When the mutation rate is low, populations will be genotypically stable and cluster at the top of the fitness peak. The variant with the highest fitness (red) will easily outcompete all others.b| When the mutation rate is high, variants spread out over their corresponding peaks. The population on the flatter peak (blue) remains near its fitness optimum and has a higher mean fitness than the population located on the steeper peak (red). The flatter population will therefore prevail in competition with the population on the higher peak. Here, fitness and robustness are contrasted to show the importance of each in determining the dynamics of RNA virus populations. As described in the text, a population can theoretically be both fit and robust and thereby occupy a tall, broad peak. However, the experimental data currently available suggest that fitness and robustness are inversely correlated.
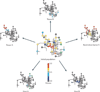
Figure 3. Using synonymous mutation to place populations in distinct fitness landscapes
a| A synonymous mutation alters the potential fitness impact of a subsequent mutation. Although two synonymous codons for arginine (such as AGG and CGG) are separated by a neutral A®C mutation, these codons differ in their propensity to mutate non-synonymously and non-conservatively. Shown are all six arginine codons (red circles) and, for each, the proportion of all potential mutations that would be non-synonymous. Synonymous mutations are indicated as solid lines, and non-synonymous mutations are indicated as dashed lines. b| Large-scale synonymous mutation preserves the consensus amino acid sequence, but relocates viral populations in sequence space. When these viruses replicate with a high mutation rate, the genetic architecture of the resultant populations differs, and the populations reside in distinct fitness landscapes. The figure shows the results of a poliovirus experiment in which the wild-type virus was compared with two variants, Max and SD, which contain 566 and 934 synonymous substitutions, respectively, in the 2,643-nucleotide sequence encoding the viral capsid protein. The SD variant was found to be the least mutationally robust (for example, it was hypersensitive to an RNA mutagen, relative to the Max variant and the wild type), suggesting that it resides on a steeper, less neutral landscape.
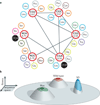
Figure 4. Dynamics of viral networks under selection in the host
The initial viral population is represented by the pipe diagram at the centre of the figure. Each individual varient of the population is represented by a ball, coloured according to fitness, and these balls are connected to each other by a genetic network. Robustness allows the population to explore an extensive region of sequence space, resulting in a larger number of individuals that are able to adapt quickly to environmental challenges. As the viral population confronts changes in the environment, the mutant distribution changes to favour those better adapted to a particular condition, such as replication in a different tissue (tissue A versus tissue B), in a different host (naive versus immunologically primed) or in a different species (called zoonotic adaptation). Robustness and diversity might also allow the viral population to overcome immunological challenges such as that of antiviral restriction factors. With each of these challenges, the fitness landscape changes and so does the distribution of mutants in the population. The majority of the variants in the initial population are expected to be poorly adapted to a new environment and to have very low fitness (grey balls). However, given the high degree of phenotypic diversity in the initial founder population, there is a high chance that one or more variants (those with a fitness greater than 0 in the new environment) will quickly adapt and propogate in the new environment, whereas those of low fitness are expected to diminish over time. For example, a variant of intermediate fitness in host A (a green ball) could eventually predominate in this environment if the genotype of this variant is better adapted to this new environment than the other genotypes present in this host. However, if the new environment is host B, then an even less fit variant (dark blue) could theoretically reach fixation as the population is exposed to a different set of selective pressures. The nature of the selective pressure in each new environment is the ultimate factor that decides the distribution of mutants in the population.
Similar articles
- RNA virus genetic robustness: possible causes and some consequences.
Elena SF. Elena SF. Curr Opin Virol. 2012 Oct;2(5):525-30. doi: 10.1016/j.coviro.2012.06.008. Epub 2012 Jul 19. Curr Opin Virol. 2012. PMID: 22818515 Review. - Costs and benefits of mutational robustness in RNA viruses.
Stern A, Bianco S, Yeh MT, Wright C, Butcher K, Tang C, Nielsen R, Andino R. Stern A, et al. Cell Rep. 2014 Aug 21;8(4):1026-36. doi: 10.1016/j.celrep.2014.07.011. Epub 2014 Aug 7. Cell Rep. 2014. PMID: 25127138 Free PMC article. - Fidelity Variants and RNA Quasispecies.
Bordería AV, Rozen-Gagnon K, Vignuzzi M. Bordería AV, et al. Curr Top Microbiol Immunol. 2016;392:303-22. doi: 10.1007/82_2015_483. Curr Top Microbiol Immunol. 2016. PMID: 26499340 Free PMC article. Review. - RNA virus population diversity: implications for inter-species transmission.
Bordería AV, Stapleford KA, Vignuzzi M. Bordería AV, et al. Curr Opin Virol. 2011 Dec;1(6):643-8. doi: 10.1016/j.coviro.2011.09.012. Epub 2011 Oct 22. Curr Opin Virol. 2011. PMID: 22440922 Review. - RNA Virus Fidelity Mutants: A Useful Tool for Evolutionary Biology or a Complex Challenge?
Kautz TF, Forrester NL. Kautz TF, et al. Viruses. 2018 Nov 1;10(11):600. doi: 10.3390/v10110600. Viruses. 2018. PMID: 30388745 Free PMC article. Review.
Cited by
- Emerging Microorganisms and Infectious Diseases: One Health Approach for Health Shared Vision.
Ristori MV, Guarrasi V, Soda P, Petrosillo N, Gurrieri F, Longo UG, Ciccozzi M, Riva E, Angeletti S. Ristori MV, et al. Genes (Basel). 2024 Jul 11;15(7):908. doi: 10.3390/genes15070908. Genes (Basel). 2024. PMID: 39062687 Free PMC article. Review. - Inhibition of porcine reproductive and respiratory syndrome virus replication by rifampicin in vitro.
Wei R, Li L, Chen H, Wang X, Chen Y, Liu X. Wei R, et al. Front Vet Sci. 2024 Jul 10;11:1439015. doi: 10.3389/fvets.2024.1439015. eCollection 2024. Front Vet Sci. 2024. PMID: 39051013 Free PMC article. - The hidden RNA viruses in Blattodea (cockroaches and termites).
Wu H, Li W, Fan J, Jiang S, Li J, Hu P, Yu Z, Li Y, Pang R, Wu H. Wu H, et al. Microb Genom. 2024 Jul;10(7):001265. doi: 10.1099/mgen.0.001265. Microb Genom. 2024. PMID: 39037207 Free PMC article. - Adenosine modifications impede SARS-CoV-2 RNA-dependent RNA transcription.
Snyder LR, Kilde I, Nemudryi A, Wiedenheft B, Koutmos M, Koutmou KS. Snyder LR, et al. RNA. 2024 Aug 16;30(9):1141-1150. doi: 10.1261/rna.079991.124. RNA. 2024. PMID: 38942480 Free PMC article. - Natural variation in neuraminidase activity influences the evolutionary potential of the seasonal H1N1 lineage hemagglutinin.
Liu T, Reiser WK, Tan TJC, Lv H, Rivera-Cardona J, Heimburger K, Wu NC, Brooke CB. Liu T, et al. Virus Evol. 2024 Jun 19;10(1):veae046. doi: 10.1093/ve/veae046. eCollection 2024. Virus Evol. 2024. PMID: 38915760 Free PMC article.
References
- Holland J, et al. Rapid evolution of RNA genomes. Science. 1982;215:1577–1585. - PubMed
- Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R. Viral mutation rates. J. Virol. 2010;84:9733–9748. A recent and comprehensive review of viral mutation rates.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources