Circulating microparticles carry a functional endothelial nitric oxide synthase that is decreased in patients with endothelial dysfunction - PubMed (original) (raw)
Circulating microparticles carry a functional endothelial nitric oxide synthase that is decreased in patients with endothelial dysfunction
Patrick Horn et al. J Am Heart Assoc. 2012.
Abstract
Background: Microparticles (MPs) are circulating membrane particles of less than a micrometer in diameter shed from endothelial and blood cells. Recent literature suggests that MPs are not just functionally inert cell debris but may possess biological functions and mediate the communication between vascular cells. As a significant proportion of MPs originate from platelets and endothelial cells, we hypothesized that MPs may harbor functional enzymes including an endothelial NO synthase (eNOS).
Methods and results: Using immunoprecipitation and Western blot analysis, we found that human circulating MPs carry an eNOS. Ca(2+) and l-arginine-dependent NOS activity of crude enzyme extract from MPs was determined by measuring the conversion of [(3)H]-L-arginine to [(3)H]-citrulline and NOS-dependent nitrite production. NOS-dependent NO production in intact MPs was assessed by the NO-specific fluorescent probe MNIP-Cu. In patients with cardiovascular disease, endothelial dysfunction was associated with an increase in the total number of circulating MPs as well as a significant decrease in the expression and activity of eNOS in MPs. No difference in reactive oxygen species was noted in MPs isolated from either group.
Conclusions: Our data further support the concept that circulating MPs may not only retain phenotypic markers but also preserve the functionality of enzymes of the cells they originate from, including eNOS.
Figures
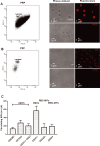
Figure 1.
Analysis of composition and morphology of different fractions of human plasma obtained by sequential centrifugation. A, PRP contains a dense population comprising platelets with dimensions >1 μm and MPs with dimensions <1 μm, as shown by flow cytometry (left panel) and phase‐contrast or fluorescence‐based laser‐scanning microscopy (middle and right panels). Membranes were stained with DiD. B, PFP contains MPs only. C, MP subpopulations in PFP were discriminated by flow‐cytometric analysis according to the expression of membrane‐specific antigens: EMPs, PMPs, RBC‐MPs, and WBC‐MPs. Values are mean±SEM. PRP indicates platelet‐rich plasma; PFP, platelet‐free plasma; MPs, microparticles; EMPs, endothelial‐derived microparticles; PMPs, platelet‐derived microparticles; RBC‐MPs, erythrocyte‐derived micoparticles; WBC‐MPs, leukocyte‐derived microparticles.
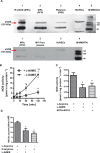
Figure 2.
MPs express a functional eNOS. A, Western blot analysis of eNOS obtained by immunoprecipitation (IP) from human MPs, crude extracts of circulating MPs, and platelets, as well as HUVECs as a control (top) and MPs, MP‐free plasma, and HUVECs (bottom). B, Crude MP lysate converted [3H]‐l‐arginine to [3H]‐l‐citrulline in a time‐dependent fashion. *P<0.0125 compared with baseline, #P<0.0125 compared with L‐NAME (2B). C, NOS activity is inhibited by the NOS inhibitor L‐NAME and by the Ca2+ chelators EDTA and EGTA. D, NOS‐dependent nitrite accumulation was substrate dependent (n=6). *P<0.025 compared with l‐arginine only. P values represent Bonferroni‐corrected significance levels. MPs indicates microparticles; eNOS, endothelial nitric oxide synthase; PRP, platelet‐rich plasma; PFP, platelet‐free plasma; HUVEC, human umbilical vein endothelial cell; MWM, molecular weight marker; L‐NAME, NG‐nitro‐l‐arginine‐methyl ester monohydrochloride.
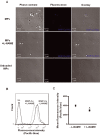
Figure 3.
Direct detection of NO in human circulating MPs. MPs loaded with the NO fluorescent probe MNIP‐Cu were highly fluorescent (CTRL), as measured by laser‐scanning microscopy (A) and flow cytometry (n=10; B+C). The addition of the specific NOS inhibitor L‐NAME decreased fluorescence. Unloaded MPs (UL) were used as a control for autofluorescence. *P<0.05 compared with samples without L‐NAME. NO indicates nitric oxide; MPs, microparticles; NOS, nitric oxide synthase; L‐NAME, NG‐nitro‐l‐arginine‐methyl ester monohydrochloride; MNIP‐Cu, 4‐methoxy‐2‐(1H‐naphtho[2,3‐d]imidazol‐2‐yl)phenol.
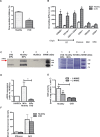
Figure 4.
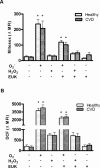
The MPs' eNOS activity is decreased in patients with endothelial dysfunction. Patients with cardiovascular disease (CVD) exhibited decreased endothelial function as assessed by flow‐mediated vasodilation (A) and increased MPs levels (especially endothelial cell– and platelet‐derived MPs) in plasma (B) compared with healthy subjects. C+D, MPs from these patients carry less eNOS protein compared with healthy controls. C, Western blot analysis, representative of n=5 independent gels (left); protein bands stained with colloidal Coomassie Brilliant Blue, confirming equal gel loading between samples (right). D, Densitometry band intensity was normalized vs a standard HUVEC lysate and was quantified using Image J software©. *P<0.017 compared with healthy subjects. E, NOS activity in MPs from CVD (l‐citrulline synthesis) was decreased compared with healthy controls. F, Mean fluorescence intensity of MPs labeled with MitoSox, a probe for O2−, or DCF, a probe for ROS, did not significantly differ between groups (measured by flow cytometry). *P<0.05 compared with healthy subjects. Values are mean±SEM. MPs indicates microparticles; eNOS, endothelial nitric oxide synthase; EC, endothelial cell; RBC, red blood cell; WBC, white blood cell; HUVEC, human umbilical vein endothelial cell; L‐NAME, NG‐nitro‐l‐arginine‐methyl ester monohydrochloride; DCF, 2′,7′‐dichlorodihydrodiclorofluorescein diacetate; ROS, reactive oxygen species.
Figure 5.
ROS levels in MPs from healthy subjects and patients with CVD. Mean fluorescence intensity of (A) MitoSox, a probe for O2−, and (B) DCF, a probe for ROS, did not significantly differ between groups. MPs were treated with PBS as a control, with an O2−‐generating system, with H2O2 or with the superoxide dismutase mimic EUK. MPs were stained with MitoSox (n=5) or with DCF diacetate (n=5) or were left unlabeled as an autofluorescence control. Probes were analyzed in a flow cytometer. *P<0.05 compared with the control. Values are mean±SEM. MPs indicates microparticles; ROS, reactive oxygen species; DCF, 2′,7′‐dichlorodihydrodiclorofluorescein diacetate; CVD, cardiovascular disease. MFI, mean fluorescence intensity; EUK‐134, chloro[[2,2'‐[1,2‐ethanediylbis[(nitrilo‐κN)methylidyne]]bis[6‐methoxy‐phenolato‐κO]]]‐manganese.
Similar articles
- Circulating microparticles from patients with valvular heart disease and cardiac surgery inhibit endothelium-dependent vasodilation.
Fu L, Hu XX, Lin ZB, Chang FJ, Ou ZJ, Wang ZP, Ou JS. Fu L, et al. J Thorac Cardiovasc Surg. 2015 Sep;150(3):666-72. doi: 10.1016/j.jtcvs.2015.05.069. Epub 2015 Jun 5. J Thorac Cardiovasc Surg. 2015. PMID: 26145768 - Role of eNOS- and NOX-containing microparticles in endothelial dysfunction in patients with obesity.
Dimassi S, Chahed K, Boumiza S, Canault M, Tabka Z, Laurant P, Riva C. Dimassi S, et al. Obesity (Silver Spring). 2016 Jun;24(6):1305-12. doi: 10.1002/oby.21508. Epub 2016 Apr 30. Obesity (Silver Spring). 2016. PMID: 27130266 - Microparticles from Patients with the Acute Coronary Syndrome Impair Vasodilatation by Inhibiting the Akt/eNOS-Hsp90 Signaling Pathway.
Han WQ, Chang FJ, Wang QR, Pan JQ. Han WQ, et al. Cardiology. 2015;132(4):252-60. doi: 10.1159/000438782. Cardiology. 2015. PMID: 26329646 - Evolving role of microparticles in the pathophysiology of endothelial dysfunction.
Lovren F, Verma S. Lovren F, et al. Clin Chem. 2013 Aug;59(8):1166-74. doi: 10.1373/clinchem.2012.199711. Epub 2013 Mar 25. Clin Chem. 2013. PMID: 23529703 Review. - Nitric Oxide and Endothelial Dysfunction.
Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Cyr AR, et al. Crit Care Clin. 2020 Apr;36(2):307-321. doi: 10.1016/j.ccc.2019.12.009. Crit Care Clin. 2020. PMID: 32172815 Free PMC article. Review.
Cited by
- Extracellular Vesicles: A Novel Target for Exercise-Mediated Reductions in Type 2 Diabetes and Cardiovascular Disease Risk.
Eichner NZM, Erdbrügger U, Malin SK. Eichner NZM, et al. J Diabetes Res. 2018 Jun 19;2018:7807245. doi: 10.1155/2018/7807245. eCollection 2018. J Diabetes Res. 2018. PMID: 30018986 Free PMC article. Review. - Platelets in Renal Disease.
Gomchok D, Ge RL, Wuren T. Gomchok D, et al. Int J Mol Sci. 2023 Sep 29;24(19):14724. doi: 10.3390/ijms241914724. Int J Mol Sci. 2023. PMID: 37834171 Free PMC article. Review. - Circulating microparticles are associated with plaque burden and cause eNOS uncoupling in patients with carotid atherosclerosis.
Han X, Li T, Wang T, Wang B, Li Y, Wang L, Lu Z, Wu A, Liu L, Pan G, Zhao M. Han X, et al. Front Pharmacol. 2022 Nov 3;13:976644. doi: 10.3389/fphar.2022.976644. eCollection 2022. Front Pharmacol. 2022. PMID: 36408271 Free PMC article. - Microparticles and cardiovascular diseases.
Voukalis C, Shantsila E, Lip GYH. Voukalis C, et al. Ann Med. 2019 May-Jun;51(3-4):193-223. doi: 10.1080/07853890.2019.1609076. Epub 2019 Jun 17. Ann Med. 2019. PMID: 31007084 Free PMC article. Review. - Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function?
Cortese-Krott MM, Kelm M. Cortese-Krott MM, et al. Redox Biol. 2014 Jan 9;2:251-8. doi: 10.1016/j.redox.2013.12.027. eCollection 2014. Redox Biol. 2014. PMID: 24494200 Free PMC article. Review.
References
- Amabile N, Rautou PE, Tedgui A, Boulanger CM. Microparticles: key protagonists in cardiovascular disorders. Semin Thromb Hemost. 2010; 36:907-916 - PubMed
- Burnier L, Fontana P, Kwak BR, Angelillo‐Scherrer A. Cell‐derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009; 101:439-451 - PubMed
- George FD. Microparticles in vascular diseases. Thromb Res. 2008; 122:55-59 - PubMed
- Garcia BA, Smalley DM, Cho H, Shabanowitz J, Ley K, Hunt DF. The platelet microparticle proteome. J Proteome Res. 2005; 4:1516-1521 - PubMed
- Smalley DM, Root KE, Cho H, Ross MM, Ley K. Proteomic discovery of 21 proteins expressed in human plasma‐derived but not platelet‐derived microparticles. Thromb Haemost. 2007; 97:67-80 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous