Monocyte chemoattractant protein-1/CCL2 produced by stromal cells promotes lung metastasis of 4T1 murine breast cancer cells - PubMed (original) (raw)
Monocyte chemoattractant protein-1/CCL2 produced by stromal cells promotes lung metastasis of 4T1 murine breast cancer cells
Teizo Yoshimura et al. PLoS One. 2013.
Abstract
MCP-1/CCL2 plays an important role in the initiation and progression of cancer. Since tumor cells produce MCP-1, they are considered to be the main source of this chemokine. Here, we examined whether MCP-1 produced by non-tumor cells affects the growth and lung metastasis of 4T1 breast cancer cells by transplanting them into the mammary pad of WT or MCP-1(-/-) mice. Primary tumors at the injected site grew similarly in both mice; however, lung metastases were markedly reduced in MCP-1(-/-) mice, with significantly longer mouse survival. High levels of MCP-1 mRNA were detected in tumors growing in WT, but not MCP-1(-/-) mice. Serum MCP-1 levels were increased in tumor-bearing WT, but not MCP-1(-/-) mice. Transplantation of MCP-1(-/-) bone marrow cells into WT mice did not alter the incidence of lung metastasis, whereas transplantation of WT bone marrow cells into MCP-1(-/-) mice increased lung metastasis. The primary tumors of MCP-1(-/-) mice consistently developed necrosis earlier than those of WT mice and showed decreased infiltration by macrophages and reduced angiogenesis. Interestingly, 4T1 cells that metastasized to the lung constitutively expressed elevated levels of MCP-1, and intravenous injection of 4T1 cells producing a high level of MCP-1 resulted in increased tumor foci in the lung of WT and MCP-1(-/-) mice. Thus, stromal cell-derived MCP-1 in the primary tumors promotes lung metastasis of 4T1 cells, but tumor cell-derived MCP-1 can also contribute once tumor cells enter the circulation. A greater understanding of the source and role of this chemokine may lead to novel strategies for cancer treatment.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
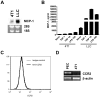
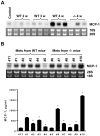
Figure 1. The expression of MCP-1 and CCR2 by 4T1 cells.
A. The expression of MCP-1 mRNA by 4T1 cells was examined and compared to that of LLC by Northern blotting. B. The concentration of MCP-1 in the culture supernatants of 4T1 or LLC cells incubated for 24 h with 1 or 10 ng/ml of murine TNFα or 100 ng/ml of LPS or without any stimulus was measured by ELISA. C. The surface expression of CCR2 on 4T1 cells was examined by FACS. D. The expression of CCR2 mRNA in 4T1 cells was examined by RT-PCR. Thioglycollate-induced mouse peritoneal exudates cells were used as control.
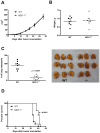
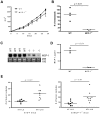
Figure 2. The absence of MCP-1 in tumor stroma reduces the lung metastasis of 4T1 cells and prolongs survival.
A. 1×105 4T1 cells were injected into a mammary pad of WT or MCP-1−/− mice. The size of each primary tumor was measured and the area was calculated. n = 9 for WT, n = 8 for MCP-1−/− mice. B. Primary tumors were excised from WT and MCP-1−/− mice 31 days after tumor cell injection and weighed. n = 9 for WT, n = 8 for MCP-1−/− mice. C. Mice were euthanized on day 31, and lungs were harvested and fixed in Bouin's solution. The number of metastatic tumor nodules on the surface of lungs of each mouse was counted by eye. n = 9 for WT, n = 8 for MCP-1−/− mice. D. Ten thousand 4T1 cells were injected in a mammary pad of each mouse and the survival of each mouse was examined. n = 8 for WT, n = 8 for MCP-1−/− mice.
Figure 3. Non-tumor cells in tumor stroma are the major source of MCP-1.
A. 1×105 4T1 cells were injected into a mammary pad of WT or MCP-1−/− mice. The expression of MCP-1 mRNA in tumors of WT or MCP-1−/− mice was examined by Northern blotting. All RNA samples were run on a single agarose gel, blotted to a single membrane. The membrane was hybridized to 32P-labeled cDNA probe and exposed to a X-ray film. B. The level of MCP-1 mRNA expression in a WT and MCP-1−/− tumor was compared with that of 4T1 cells incubated in vitro with or without proinflammatory stimuli by Northern blotting. C. The serum MCP-1 concentration of tumor-bearing mice 7, 14, 21 or 28 days after tumor cell inoculation was measured by ELISA. n = 3 per each group at each time point. *p<0.05. D. 1×105 4T1 cells were injected into a mammary pad of each mouse. All mice were euthanized 4 weeks after the injection and lungs were inflated with Bouin's solution and fixed in the same solution. The number of metastatic tumor nodules on the surface of the lung of each mouse was counted by eye. n = 4 for each group. E. BM cells were collected from femurs of mice belonging to each group and genomic DNA was extracted. Ten µg of genomic DNA was digested with _Bam_HI and then subjected to Southern blot analysis. The 7.5-kb WT allele and the 5.5-kb mutant allele are indicated by arrow heads.
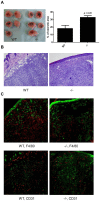
Figure 4. MCP-1-deficiency in tumor stroma results in early necrosis, reduced macrophage infiltration and reduced angiogenesis.
A. 1×105 4T1 cells were injected into a mammary pad of WT or MCP-1−/− mice. Primary tumors were excised two weeks after 4T1 cell injection and fixed in formalin. Tissue sections were prepared from the center of each tumor, stained by H&E, and the area of necrosis was measured. % of necrotic area was calculated by using a formula, area of necrosis/area of tumor×100. n = 5 for each group. B. H&E section of tumor tissue away from necrosis. C. Immunohistochemical examination of tumor sections. Green, cytokeratin; Red, F4/80 or CD31.
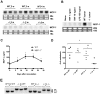
Figure 5. 4T1 cells that metastasized to the lung express higher level of MCP-1.
A. 1×105 4T1 cells were injected into a mammary pad of WT or MCP-1−/− mice. Total RNA was extracted from the lung of tumor-bearing WT or MCP-1−/− mice and the expression of MCP-1 mRNA was examined by Northern blotting. B. Total RNA was extracted from 4T1 cells obtained from metastatic lung tumors of WT or MCP-1−/− mice and the expression of MCP-1 mRNA was evaluated by Northern blotting (upper panel). 1×105 cells were seeded into 12-well plates and incubated at 37°C overnight. Medium was replaced by 1 ml fresh medium and incubated for an additional 24 hrs. Cell-free culture supernatants were collected and the concentrations of MCP-1 were measured by ELISA (lower panel). * p<0.01, n = 2.
Figure 6. Increased MCP-1 expression in 4T1 cells has no effect on spontaneous lung metastasis in MCP-1−/− mice, but increases lung metastasis after intravenous injection.
A. 1×105 4T1-L10 cells were injected into a mammary pad of WT or MCP-1−/− mice. The size of each primary tumor was measured and the area was calculated. n = 4 for WT, n = 3 for MCP-1−/− mice. B. 1×105 4T1-L10 cells were injected into a mammary pad of WT or MCP-1−/− mice. All mice were euthanized 4 weeks after the injection and the number of metastatic tumor nodules on the surface of each lung was counted by eye. C. Total RNA was extracted from each tumor and the expression of MCP-1 mRNA was examined by Northern analysis. Ten µg of total RNA was used. D. Sera were collected 2 weeks after the injection of 4T1-L10 cells and MCP-1 concentrations were measured by ELISA. E. 4T1-L5 or 4T1-L10 cells (5×104 cells in 0.2 ml PBS) were intravenously injected into WT (left panel) or MCP-1−/− mice (right panel). Two weeks later, mice were euthanized and the number of metastatic tumor nodules on the lung was counted. The results are the summary of two independent experiments. n = 8 for WT, and n = 8 (4T1-L5) or n = 9 (4Y1-L10) for MCP-1−/− mice.
Similar articles
- Crosstalk between Cancer Cells and Fibroblasts for the Production of Monocyte Chemoattractant Protein-1 in the Murine 4T1 Breast Cancer.
Imamura M, Li T, Li C, Fujisawa M, Mukaida N, Matsukawa A, Yoshimura T. Imamura M, et al. Curr Issues Mol Biol. 2021 Oct 22;43(3):1726-1740. doi: 10.3390/cimb43030122. Curr Issues Mol Biol. 2021. PMID: 34698088 Free PMC article. - Ethanol promotes mammary tumor growth and angiogenesis: the involvement of chemoattractant factor MCP-1.
Wang S, Xu M, Li F, Wang X, Bower KA, Frank JA, Lu Y, Chen G, Zhang Z, Ke Z, Shi X, Luo J. Wang S, et al. Breast Cancer Res Treat. 2012 Jun;133(3):1037-48. doi: 10.1007/s10549-011-1902-7. Epub 2011 Dec 9. Breast Cancer Res Treat. 2012. PMID: 22160640 Free PMC article. - The Yin/Yan of CCL2: a minor role in neutrophil anti-tumor activity in vitro but a major role on the outgrowth of metastatic breast cancer lesions in the lung in vivo.
Lavender N, Yang J, Chen SC, Sai J, Johnson CA, Owens P, Ayers GD, Richmond A. Lavender N, et al. BMC Cancer. 2017 Jan 31;17(1):88. doi: 10.1186/s12885-017-3074-2. BMC Cancer. 2017. PMID: 28143493 Free PMC article. - The chemokine monocyte chemoattractant protein-1/CCL2 is a promoter of breast cancer metastasis.
Yoshimura T, Li C, Wang Y, Matsukawa A. Yoshimura T, et al. Cell Mol Immunol. 2023 Jul;20(7):714-738. doi: 10.1038/s41423-023-01013-0. Epub 2023 May 19. Cell Mol Immunol. 2023. PMID: 37208442 Free PMC article. Review. - The chemokine MCP-1 (CCL2) in the host interaction with cancer: a foe or ally?
Yoshimura T. Yoshimura T. Cell Mol Immunol. 2018 Apr;15(4):335-345. doi: 10.1038/cmi.2017.135. Epub 2018 Jan 29. Cell Mol Immunol. 2018. PMID: 29375123 Free PMC article. Review.
Cited by
- Cancer Cell-Derived Granulocyte-Macrophage Colony-Stimulating Factor Is Dispensable for the Progression of 4T1 Murine Breast Cancer.
Yoshimura T, Nakamura K, Li C, Fujisawa M, Shiina T, Imamura M, Li T, Mukaida N, Matsukawa A. Yoshimura T, et al. Int J Mol Sci. 2019 Dec 16;20(24):6342. doi: 10.3390/ijms20246342. Int J Mol Sci. 2019. PMID: 31888216 Free PMC article. - Cadherin-12 enhances proliferation in colorectal cancer cells and increases progression by promoting EMT.
Ma J, Zhao J, Lu J, Wang P, Feng H, Zong Y, Ou B, Zheng M, Lu A. Ma J, et al. Tumour Biol. 2016 Jul;37(7):9077-88. doi: 10.1007/s13277-015-4555-z. Epub 2016 Jan 14. Tumour Biol. 2016. PMID: 26762412 Free PMC article. - CCL2 in the Tumor Microenvironment.
O'Connor T, Heikenwalder M. O'Connor T, et al. Adv Exp Med Biol. 2021;1302:1-14. doi: 10.1007/978-3-030-62658-7_1. Adv Exp Med Biol. 2021. PMID: 34286437 - Induction of Monocyte Chemoattractant Proteins in Macrophages via the Production of Granulocyte/Macrophage Colony-Stimulating Factor by Breast Cancer Cells.
Yoshimura T, Imamichi T, Weiss JM, Sato M, Li L, Matsukawa A, Wang JM. Yoshimura T, et al. Front Immunol. 2016 Jan 20;7:2. doi: 10.3389/fimmu.2016.00002. eCollection 2016. Front Immunol. 2016. PMID: 26834744 Free PMC article. - Non-Myeloid Cells are Major Contributors to Innate Immune Responses via Production of Monocyte Chemoattractant Protein-1/CCL2.
Yoshimura T, Galligan C, Takahashi M, Chen K, Liu M, Tessarollo L, Wang JM. Yoshimura T, et al. Front Immunol. 2014 Jan 7;4:482. doi: 10.3389/fimmu.2013.00482. eCollection 2014 Jan 7. Front Immunol. 2014. PMID: 24432017 Free PMC article.
References
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454: 436–444. - PubMed
- Bottazzi B, Colotta F, Sica A, Nobili N, Mantovani A (1990) A chemoattractant expressed in human sarcoma cells (tumor-derived chemotactic factor, TDCF) is identical to monocyte chemoattractant protein-1/monocyte chemotactic and activating factor (MCP-1/MCAF). Int J Cancer 45: 795–797. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous