Structure of a dimeric crenarchaeal Cas6 enzyme with an atypical active site for CRISPR RNA processing - PubMed (original) (raw)
Structure of a dimeric crenarchaeal Cas6 enzyme with an atypical active site for CRISPR RNA processing
Judith Reeks et al. Biochem J. 2013.
Abstract
The competition between viruses and hosts is played out in all branches of life. Many prokaryotes have an adaptive immune system termed 'CRISPR' (clustered regularly interspaced short palindromic repeats) which is based on the capture of short pieces of viral DNA. The captured DNA is integrated into the genomic DNA of the organism flanked by direct repeats, transcribed and processed to generate crRNA (CRISPR RNA) that is loaded into a variety of effector complexes. These complexes carry out sequence-specific detection and destruction of invading mobile genetic elements. In the present paper, we report the structure and activity of a Cas6 (CRISPR-associated 6) enzyme (Sso1437) from Sulfolobus solfataricus responsible for the generation of unit-length crRNA species. The crystal structure reveals an unusual dimeric organization that is important for the enzyme's activity. In addition, the active site lacks the canonical catalytic histidine residue that has been viewed as an essential feature of the Cas6 family. Although several residues contribute towards catalysis, none is absolutely essential. Coupled with the very low catalytic rate constants of the Cas6 family and the plasticity of the active site, this suggests that the crRNA recognition and chaperone-like activities of the Cas6 family should be considered as equal to or even more important than their role as traditional enzymes.
Figures
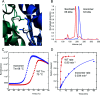
Figure 1. SsoCas6 cleaves a CRISPR RNA repeat
(A) Representative time course of RNA cleavage by SsoCas6 at 60°C under single-turnover conditions, analysed by gel electrophoresis and phosphorimaging. Labels ‘s’ and ‘p’ indicate substrates and products respectively. The control reaction ‘c’ was carried out in the absence of SsoCas6 for 25 min. (B) Single-turnover kinetic rates for cleavage of a CRISPR repeat RNA by SsoCas6 as a function of reaction temperature. The sequence of the RNA oligonucleotide substrate is shown at the top with the cleavage position indicated with an arrow. Each rate was calculated from at least six data points as described in the Experimental section, with means±S.E.M. calculated from curve fitting shown.
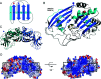
Figure 2. The crystal structure of SsoCas6
(A) Schematic representation of a typical ferredoxin-like fold with the β-strands as blue arrows and the α-helices as cyan cylinders. The N- and C-termini are shown as blue and red spheres respectively. (B) The structure of SsoCas6 with secondary-structure elements labelled. Disordered loops are shown as broken black lines and the glycine-rich loop shown in yellow. The location of the missing α-helix of the N-terminal domain is indicated. (C) View of the SsoCas6 dimer. (D) Electrostatic surface potential of the SsoCas6 dimer generated using CCP4MG [50]. The black boxes indicate the active-site region, with the broken box indicating the active site on the non-visible face of the dimer.
Figure 3. Dimerization of SsoCas6
(A) View of Leu170 and Val202 at the dimer interface. These residues were each changed to aspartate to disrupt the interface. (B) Gel-filtration elution profiles of dimeric SsoCas6 and the SsoCas6-L170D/V202D variant, which elutes with a retention volume consistent with a monomeric composition (expected molecular mass of 33 kDa). (C) Thermofluor analysis of heat-induced denaturation of wild-type (WT) and monomeric SsoCas6, showing the 7°C difference in melting temperatures (Tm). (D) Single-turnover kinetic comparison of wild-type and monomeric SsoCas6. The catalytic activity of the monomer is reduced by 95% compared with the wild-type (WT) enzyme.
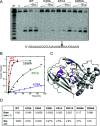
Figure 4. Delineating the SsoCas6 active site
(A) Phosphorimage of a denaturing polyacrylamide gel showing the reaction products of repeat RNA incubated with wild-type (WT) and selected variant Cas6 enzymes. Lane m shows an RNA ladder generated by alkaline hydrolysis and lane c shows RNA incubated for 60 min in the absence of protein. Time points correspond to 1, 5 and 50 min incubations at 60°C. The cleavage site is indicated in the RNA sequence below the image. (B) Plot of the reaction kinetics for selected variants of SsoCas6 (red, wild-type, WT; black, S268A; green, K51A; blue, K28A). All data points were measured in triplicate and are means±S.E.M. (C) Structure of the SsoCas6 monomer. The glycine-rich loop is shown in yellow to highlight the approximate position of the active site. The positions of selected side chains targeted by site-directed mutagenesis are shown as magenta sticks. Of these residues, it was not possible to define the absolute conformation of the side chains of Lys25, Lys28 and Lys51 from the electron density. Chains B and D are superimposed on chain A to include all of the desired residues. (D) First-order rate constants for wild-type and selected variant SsoCas6 enzymes. Relative activity (Rel. act.) is expressed as a percentage of wild-type (WT) activity.
Similar articles
- Structure of the archaeal Cascade subunit Csa5: relating the small subunits of CRISPR effector complexes.
Reeks J, Graham S, Anderson L, Liu H, White MF, Naismith JH. Reeks J, et al. RNA Biol. 2013 May;10(5):762-9. doi: 10.4161/rna.23854. Epub 2013 Apr 22. RNA Biol. 2013. PMID: 23846216 Free PMC article. - A complex of Cas proteins 5, 6, and 7 is required for the biogenesis and stability of clustered regularly interspaced short palindromic repeats (crispr)-derived rnas (crrnas) in Haloferax volcanii.
Brendel J, Stoll B, Lange SJ, Sharma K, Lenz C, Stachler AE, Maier LK, Richter H, Nickel L, Schmitz RA, Randau L, Allers T, Urlaub H, Backofen R, Marchfelder A. Brendel J, et al. J Biol Chem. 2014 Mar 7;289(10):7164-7177. doi: 10.1074/jbc.M113.508184. Epub 2014 Jan 23. J Biol Chem. 2014. PMID: 24459147 Free PMC article. - Structure and mechanism of the CMR complex for CRISPR-mediated antiviral immunity.
Zhang J, Rouillon C, Kerou M, Reeks J, Brugger K, Graham S, Reimann J, Cannone G, Liu H, Albers SV, Naismith JH, Spagnolo L, White MF. Zhang J, et al. Mol Cell. 2012 Feb 10;45(3):303-13. doi: 10.1016/j.molcel.2011.12.013. Epub 2012 Jan 5. Mol Cell. 2012. PMID: 22227115 Free PMC article. - Hot and crispy: CRISPR-Cas systems in the hyperthermophile Sulfolobus solfataricus.
Zhang J, White MF. Zhang J, et al. Biochem Soc Trans. 2013 Dec;41(6):1422-6. doi: 10.1042/BST20130031. Biochem Soc Trans. 2013. PMID: 24256231 Review. - Electron microscopy studies of Type III CRISPR machines in Sulfolobus solfataricus.
Cannone G, Webber-Birungi M, Spagnolo L. Cannone G, et al. Biochem Soc Trans. 2013 Dec;41(6):1427-30. doi: 10.1042/BST20130166. Biochem Soc Trans. 2013. PMID: 24256232 Review.
Cited by
- Annotation and Classification of CRISPR-Cas Systems.
Makarova KS, Koonin EV. Makarova KS, et al. Methods Mol Biol. 2015;1311:47-75. doi: 10.1007/978-1-4939-2687-9_4. Methods Mol Biol. 2015. PMID: 25981466 Free PMC article. Review. - Origins and evolution of CRISPR-Cas systems.
Koonin EV, Makarova KS. Koonin EV, et al. Philos Trans R Soc Lond B Biol Sci. 2019 May 13;374(1772):20180087. doi: 10.1098/rstb.2018.0087. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30905284 Free PMC article. Review. - Cas6 specificity and CRISPR RNA loading in a complex CRISPR-Cas system.
Sokolowski RD, Graham S, White MF. Sokolowski RD, et al. Nucleic Acids Res. 2014 Jun;42(10):6532-41. doi: 10.1093/nar/gku308. Epub 2014 Apr 20. Nucleic Acids Res. 2014. PMID: 24753403 Free PMC article. - Structure of the CRISPR interference complex CSM reveals key similarities with cascade.
Rouillon C, Zhou M, Zhang J, Politis A, Beilsten-Edmands V, Cannone G, Graham S, Robinson CV, Spagnolo L, White MF. Rouillon C, et al. Mol Cell. 2013 Oct 10;52(1):124-34. doi: 10.1016/j.molcel.2013.08.020. Mol Cell. 2013. PMID: 24119402 Free PMC article. - CRISPR interference: a structural perspective.
Reeks J, Naismith JH, White MF. Reeks J, et al. Biochem J. 2013 Jul 15;453(2):155-66. doi: 10.1042/BJ20130316. Biochem J. 2013. PMID: 23805973 Free PMC article. Review.
References
- Mojica F. J., Diez-Villasenor C., Garcia-Martinez J., Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005;60:174–182. - PubMed
- Bolotin A., Quinquis B., Sorokin A., Ehrlich S. D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–2561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/G011400/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K000314/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources