Diacylglycerol kinases: regulated controllers of T cell activation, function, and development - PubMed (original) (raw)
Review
Diacylglycerol kinases: regulated controllers of T cell activation, function, and development
Rohan P Joshi et al. Int J Mol Sci. 2013.
Abstract
Diacylglycerol kinases (DGKs) are a diverse family of enzymes that catalyze the conversion of diacylglycerol (DAG), a crucial second messenger of receptor-mediated signaling, to phosphatidic acid (PA). Both DAG and PA are bioactive molecules that regulate a wide set of intracellular signaling proteins involved in innate and adaptive immunity. Clear evidence points to a critical role for DGKs in modulating T cell activation, function, and development. More recently, studies have elucidated factors that control DGK function, suggesting an added complexity to how DGKs act during signaling. This review summarizes the available knowledge of the function and regulation of DGK isoforms in signal transduction with a particular focus on T lymphocytes.
Figures
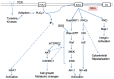
Figure 1
Diacylglycerol kinases (DGK) isoforms are divided into five subtypes based on domains apart from the C1 and catalytic domains. RVH: recoverin homology domain. PH: pleckstrin homology domain. SAM: sterile α motif. M: MARCKS homology domain. ANK, ankyrin repeat domain; PDZ-bind, PDZ-binding domain; Note, domain sizes are not to scale.
Figure 2
Diacylglycerol has a central role in TCR signaling. Engagement of the TCR leads to the activation of tyrosine kinases and the formation of a multimolecular complex including adapter proteins. PLCγ1 is recruited to and activated by this complex and in turn hydrolyses the phospholipid PIP2 to form membrane-diffusible diacylglycerol (DAG) and cytosolic IP3. DAG activates proteins including RasGRP1, PKCs, and PKD. Activation of RasGRP1 leads to Ras and AKT pathway activation. Activation of PKCs leads to the activation of NF-κB and cytoskeletal repolarization. Activation of PKD leads to integrin activation. IP3, Ras, and PKC-θ signaling cooperate to promote T cell activation through the transcription factors NFAT, AP-1, and NF-κB, respectively. Diacylglycerol kinases (DGKs) phosphorylate DAG to form phosphatidic acid (PA), thereby potentially regulating a broad set of signaling pathways downstream of the TCR.
Similar articles
- Role of Diacylglycerol Kinases in Glucose and Energy Homeostasis.
Massart J, Zierath JR. Massart J, et al. Trends Endocrinol Metab. 2019 Sep;30(9):603-617. doi: 10.1016/j.tem.2019.06.003. Epub 2019 Jul 19. Trends Endocrinol Metab. 2019. PMID: 31331711 Review. - Role of diacylglycerol kinases in T cell development and function.
Krishna S, Zhong X. Krishna S, et al. Crit Rev Immunol. 2013;33(2):97-118. doi: 10.1615/critrevimmunol.2013006696. Crit Rev Immunol. 2013. PMID: 23582058 Free PMC article. Review. - Diacylglycerol kinases in immune cell function and self-tolerance.
Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Zhong XP, et al. Immunol Rev. 2008 Aug;224:249-64. doi: 10.1111/j.1600-065X.2008.00647.x. Immunol Rev. 2008. PMID: 18759932 Free PMC article. Review. - The ζ isoform of diacylglycerol kinase plays a predominant role in regulatory T cell development and TCR-mediated ras signaling.
Joshi RP, Schmidt AM, Das J, Pytel D, Riese MJ, Lester M, Diehl JA, Behrens EM, Kambayashi T, Koretzky GA. Joshi RP, et al. Sci Signal. 2013 Nov 26;6(303):ra102. doi: 10.1126/scisignal.2004373. Sci Signal. 2013. PMID: 24280043 Free PMC article. - Diacylglycerol kinases: Relationship to other lipid kinases.
Ma Q, Gabelli SB, Raben DM. Ma Q, et al. Adv Biol Regul. 2019 Jan;71:104-110. doi: 10.1016/j.jbior.2018.09.014. Epub 2018 Sep 28. Adv Biol Regul. 2019. PMID: 30348515 Free PMC article. Review.
Cited by
- Diacylglycerol kinase is a keystone regulator of signaling relevant to the pathophysiology of asthma.
Hernandez-Lara MA, Richard J, Deshpande DA. Hernandez-Lara MA, et al. Am J Physiol Lung Cell Mol Physiol. 2024 Jul 1;327(1):L3-L18. doi: 10.1152/ajplung.00091.2024. Epub 2024 May 14. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38742284 Review. - Global Transcriptomic Profiling of Innate and Adaptive Immunity During Aspergillus flavus Endophthalmitis in a Murine Model.
Khapuinamai A, Rudraprasad D, Pandey S, Gandhi J, Mishra DK, Joseph J. Khapuinamai A, et al. Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):44. doi: 10.1167/iovs.65.4.44. Invest Ophthalmol Vis Sci. 2024. PMID: 38687493 Free PMC article. - Predicting small molecule binding pockets on diacylglycerol kinases using chemoproteomics and AlphaFold.
Mendez R, Shaikh M, Lemke MC, Yuan K, Libby AH, Bai DL, Ross MM, Harris TE, Hsu KL. Mendez R, et al. RSC Chem Biol. 2023 May 15;4(6):422-430. doi: 10.1039/d3cb00057e. eCollection 2023 Jun 7. RSC Chem Biol. 2023. PMID: 37292058 Free PMC article. - Negative intracellular regulators of T-cell receptor (TCR) signaling as potential antitumor immunotherapy targets.
Laletin V, Bernard PL, Costa da Silva C, Guittard G, Nunes JA. Laletin V, et al. J Immunother Cancer. 2023 May;11(5):e005845. doi: 10.1136/jitc-2022-005845. J Immunother Cancer. 2023. PMID: 37217244 Free PMC article. Review. - Lymphocytic Choriomeningitis Virus Clone 13 Infection Results in CD8 T Cell-Mediated Host Mortality in Diacylglycerol Kinase α-Deficient Mice.
Kudek MR, Xin G, Alson D, Holzhauer S, Shen J, Kasmani MY, Riese M, Cui W. Kudek MR, et al. J Immunol. 2023 May 1;210(9):1281-1291. doi: 10.4049/jimmunol.2101011. J Immunol. 2023. PMID: 36920384 Free PMC article.
References
- Topham M.K., Bunting M., Zimmerman G.A., McIntyre T.M., Blackshear P.J., Prescott S.M. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-ζ. Nature. 1998;394:697–700. - PubMed
- Sugiura M., Kono K., Liu H., Shimizugawa T., Minekura H., Spiegel S., Kohama T. Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization. J. Biol. Chem. 2002;277:23294–23300. - PubMed
- Merida I., Avila-Flores A., Merino E. Diacylglycerol kinases: At the hub of cell signalling. Biochem. J. 2008;410:631–631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources