Heritable remodeling of yeast multicellularity by an environmentally responsive prion - PubMed (original) (raw)
Heritable remodeling of yeast multicellularity by an environmentally responsive prion
Daniel L Holmes et al. Cell. 2013.
Abstract
Prion proteins undergo self-sustaining conformational conversions that heritably alter their activities. Many of these proteins operate at pivotal positions in determining how genotype is translated into phenotype. But the breadth of prion influences on biology and their evolutionary significance are just beginning to be explored. We report that a prion formed by the Mot3 transcription factor, [MOT3(+)], governs the acquisition of facultative multicellularity in the budding yeast Saccharomyces cerevisiae. The traits governed by [MOT3(+)] involved both gains and losses of Mot3 regulatory activity. [MOT3(+)]-dependent expression of FLO11, a major determinant of cell-cell adhesion, produced diverse lineage-specific multicellular phenotypes in response to nutrient deprivation. The prions themselves were induced by ethanol and eliminated by hypoxia-conditions that occur sequentially in the natural respiro-fermentative cycles of yeast populations. These data demonstrate that prions can act as environmentally responsive molecular determinants of multicellularity and contribute to the natural morphological diversity of budding yeast.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
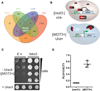
Figure 1. Prions Converge on the Principal Determinant of Yeast Multicellularity FLO11
(A) Venn diagram demonstrating the overlap in the regulons of known prion-forming yeast transcription factors. Only two genes, FLO11 and HXT2, are regulated by all four. (B) A genetic reporter for [MOT3+]. Mot3 represses transcription of DAN1 under normal growth conditions. When the DAN1 ORF is replaced with URA3, laboratory yeast strains can become prototrophic for uracil by prion-driven inactivation of Mot3. (C) Cells harboring the reporter for [MOT3+] were transformed with either empty vector (E.V.) or a galactose-inducible vector encoding Mot3. Following 24 hr of growth in galactose, 5-fold serial dilutions of cells were plated to media lacking uracil (– Uracil) to select for those that contained stably inactivated Mot3. Cells plated to uracil-containing media (+ Uracil), at the highest dilution, are also shown. Transient overexpression of Mot3 strongly induces conversion to a Ura+ phenotype. (D) qRT-PCR using mRNA isolated from isogenic [MOT3+] and [_mot3_−] colonies reveals that [MOT3+] cells have increased FLO11 expression. Values are normalized to ACT1. Error bars represent SD from triplicate colonies. See also Figure S1 and Tables S1 and S2.
Figure 2. [MOT3+] Enables Facultative Multicellular Growth
(A) Schematics on the left depict multicellular phenotypes (dark cells) that are induced by each of the common growth conditions indicated. On plates containing only proline as a nitrogen source, [MOT3+] cells formed invasive filaments that penetrated the agar surface (top; invasive growth remains after dislodging surface cells under running water). On plates containing only ethanol as a carbon source, [MOT3+] cells formed complex colony morphologies (middle). The schematic for colony morphology is based on Váchová et al. (2011). When grown to saturation in liquid media, [MOT3+] cells exhibited a decreased tendency to flocculate (bottom). Each of these behaviors of [MOT3+] cells was eliminated by treating the cells transiently with GdHCl, an inhibitor of the prion-partitioning factor Hsp104. (B) [MOT3+] cells form elaborate, biofilm-like structures when grown on semisolid ethanol media. Scale bars, 1 cm. See also Figure S2.
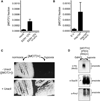
Figure 3. The N-Terminal Region Is Required for Prion-Mediated Inactivation of Mot3
(A) A schematic of full-length Mot3 and two functionally distinct truncation mutants. Regions of high predicted prion-forming propensity are indicated in red. The C-terminal nonprion-like region of Mot3 contains two C2H2 zinc fingers, indicated in blue, which are involved in DNA binding. The prion-determining region, “PrD,” as initially defined by Alberti et al. (2009) encompasses the N-terminal 295 residues of the protein. A poly-asparagine (poly-N) tract stretches from residues 143–157. In the present study, we construct a variant, “ΔPrD,” that lacks much of the PrD including the poly-N tract. An endogenous hexa-histidine motif is indicated in green. (B) Ectopic expression of ΔPrD (↑ΔPrD) from a constitutive promoter (TEF1) suppresses the Ura+ phenotype of [MOT3+]. In contrast, [MOT3+] cells ectopically expressing full-length Mot3 (↑WT) remain Ura+. This is because WT Mot3, but not ΔPrD, accumulates as SDS-resistant aggregates, as visualized by SDD-AGE and immunoblotting against the endogenous hexa-histidine motif. Amyloids from endogenous Mot3 are too low abundance to be detected in this exposure. An SDS-PAGE blot of lysates boiled in 2% LDS demonstrates comparable expression of the Mot3 variants.
Figure 4. Prion-Mediated Inactivation of Mot3 Is Required but Is Not Sufficient to Convey the Full Spectrum of [MOT3+] Phenotypes
(A) Ectopic expression of ΔPrD (↑ΔPrD) from a constitutive promoter (TEF1) suppresses the complex colony morphology, invasion, and hypo-flocculation phenotypes of [MOT3+] cells. In contrast, [MOT3+] cells that ectopically express full-length Mot3 (↑WT) retain [MOT3+] phenotypes. (B) Genetic deletion of MOT3 confers some phenotypes that mimic those of [MOT3+], including biofilm formation and invasion. Δ_mot3_ cells do not flocculate when grown to saturation in liquid media. (C) Genetic deletion of MOT3 is not sufficient to fully derepress the DAN1 promoter (P_DAN1_-URA3). Unlike [MOT3+] cells (which are Ura+), Δ_mot3_ cells partition dynamically between fully repressed (ura−) and derepressed (Ura+) states. (D) Ectopic expression of WT Mot3 stabilizes the derepressed (Ura+) state, whereas the non-aggregating variant of Mot3, ΔPrD, restores the fully repressed (ura−) state. Expression of PrD or empty vector has no effect. Duplicate transformants are shown, and serial dilutions were made onto the same plate. Bottom view is a western blot comparing expression levels of the Mot3 variants. (E) In a hypothetical model for the gain of function of Mot3 prion conformers, Mot3 interacts with another protein that corepresses transcription from the DAN1 promoter. In the [MOT3+] state, Mot3 prion particles sequester this protein, resulting in the full derepression of the DAN1 promoter. In cells altogether lacking Mot3, this protein can still bind to and partially repress the DAN1 promoter. Scale bars, 1 cm.
Figure 5. Environmental Conditions Govern Mot3 Prion Switching
(A) The spontaneous switching of Mot3 to its prion state is increased by ethanol stress. Diploid [_mot3_−] cells, or as a negative control, WT/ΔPrD heterozygotes, were incubated for 6 hr in media alone (mock), or media containing 12% ethanol (EtOH), prior to plating to media lacking uracil. Ura+ colonies were counted after 4 days and normalized by the number of total colony-forming units. Data represent mean ± SD from three independent experiments. (B) [_mot3_−] cells were transformed with either empty vector or a plasmid expressing Hsp104 from a strong constitutive promoter (GPD). Transformants were inoculated to rich media overnight, and then the fraction of [MOT3+] cells was determined as in (A). (C) Passaging [MOT3+] cells under hypoxic conditions reverts them to [_mot3_−]. (D) The effect of hypoxia is specific to [MOT3+]. A strain harboring prion states of three different proteins ([MOT3+], [_PSI+_], and [RNQ+]) was treated transiently with either GdHCl or hypoxia. Amyloids representing each prion state were detected by SDD-AGE. GdHCl, which targets the prion-partitioning factor Hsp104, eliminated all three prions, whereas hypoxia specifically eliminated [MOT3+]. See also Figure S3.
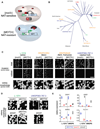
Figure 6. [MOT3+] Phenotypes Are Dictated by Genetic Variation
(A) A genetic reporter for [MOT3+] in prototrophic strains. A gene encoding resistance to the antibiotic nourseothricin (NAT) was placed under control of the DAN1 promoter, such that yeast carrying the reporter are NAT resistant only when they are [MOT3+]. (B) The reporter was integrated into genetically and ecologically diverse strains of S. cerevisiae. Each node on the phylogenetic tree (adapted from Liti et al., 2009) represents a different yeast strain that could be induced to form a NAT-resistant phenotype by overexpressed Mot3 PrD. Labels denote strains that were evaluated for [MOT3+]-dependent multicellular phenotypes. Green labels indicate that [MOT3+] produced multiple robust multicellular phenotypes, yellow indicates more limited effects of [MOT3+], and red indicates no observable effect. The two strains labeled in blue exhibited [MOT3+]-dependent changes in flocculation. (C) [MOT3+] produces a different array of multicellular phenotypes in each strain, as demonstrated by invasive behaviors and colony morphologies in four divergent strains. ferm., fermenting. (D) [MOT3+]-dependent morphological differences between L-1374 and UWOPS83-787.3 are reproduced by deleting MOT3. [MOT3+]-dependent multicellularity in L-1374 requires FLO11. (E) Demonstrative qRT-PCR analyses of the expression of Mot3-regulated genes for isogenic [MOT3+], [mot3_−], and Δ_mot3 cells in L-1374 and UWOPS83-787.3. The direct effect of Δ_mot3_ on FLO11 was not determined. Error bars represent SD from separate experiments. Scale bars, 1 cm. See also Figure S4 and Tables S4 and S5.
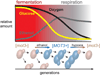
Figure 7. Model for a Function for Mot3 Prion Switching in the Respiro-Fermentative Cycle of Wine Yeasts
Yeast cells begin the cycle upon inoculation to glucose-rich grape must. Glucose is fermented to ethanol during the exponential growth phase. The ensuing ethanol stress triggers prion conversion to [MOT3+], which, in conjunction with glucose exhaustion, drives some of the cells into a multicellular growth program that protects them from stress and/or increases metabolic efficiency. As the population respires ethanol and remaining sugars, oxygen levels decline resulting in the accelerated reappearance of [_mot3_−] cells within [MOT3+] subpopulations.
Similar articles
- The sensitive [SWI (+)] prion: new perspectives on yeast prion diversity.
Hines JK, Craig EA. Hines JK, et al. Prion. 2011 Jul-Sep;5(3):164-8. doi: 10.4161/pri.5.3.16895. Epub 2011 Jul 1. Prion. 2011. PMID: 21811098 Free PMC article. - A brief overview of the Swi1 prion-[SWI+].
Goncharoff DK, Du Z, Li L. Goncharoff DK, et al. FEMS Yeast Res. 2018 Sep 1;18(6):foy061. doi: 10.1093/femsyr/foy061. FEMS Yeast Res. 2018. PMID: 29905794 Free PMC article. Review. - A systematic survey identifies prions and illuminates sequence features of prionogenic proteins.
Alberti S, Halfmann R, King O, Kapila A, Lindquist S. Alberti S, et al. Cell. 2009 Apr 3;137(1):146-58. doi: 10.1016/j.cell.2009.02.044. Cell. 2009. PMID: 19345193 Free PMC article. - Prion-like proteins as epigenetic devices of stress adaptation.
Oamen HP, Lau Y, Caudron F. Oamen HP, et al. Exp Cell Res. 2020 Nov 1;396(1):112262. doi: 10.1016/j.yexcr.2020.112262. Epub 2020 Sep 5. Exp Cell Res. 2020. PMID: 32896568 Review. - Prions are a common mechanism for phenotypic inheritance in wild yeasts.
Halfmann R, Jarosz DF, Jones SK, Chang A, Lancaster AK, Lindquist S. Halfmann R, et al. Nature. 2012 Feb 15;482(7385):363-8. doi: 10.1038/nature10875. Nature. 2012. PMID: 22337056 Free PMC article.
Cited by
- A glass menagerie of low complexity sequences.
Halfmann R. Halfmann R. Curr Opin Struct Biol. 2016 Jun;38:18-25. doi: 10.1016/j.sbi.2016.05.002. Epub 2016 May 31. Curr Opin Struct Biol. 2016. PMID: 27258703 Free PMC article. Review. - Evolutionary behaviour of bacterial prion-like proteins.
Harrison PM. Harrison PM. PLoS One. 2019 Mar 5;14(3):e0213030. doi: 10.1371/journal.pone.0213030. eCollection 2019. PLoS One. 2019. PMID: 30835736 Free PMC article. - Pernicious pathogens or expedient elements of inheritance: the significance of yeast prions.
Byers JS, Jarosz DF. Byers JS, et al. PLoS Pathog. 2014 Apr 10;10(4):e1003992. doi: 10.1371/journal.ppat.1003992. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24722628 Free PMC article. Review. No abstract available. - Protein assembly systems in natural and synthetic biology.
Chiesa G, Kiriakov S, Khalil AS. Chiesa G, et al. BMC Biol. 2020 Mar 26;18(1):35. doi: 10.1186/s12915-020-0751-4. BMC Biol. 2020. PMID: 32216777 Free PMC article. Review. - Genetic interactions involving five or more genes contribute to a complex trait in yeast.
Taylor MB, Ehrenreich IM. Taylor MB, et al. PLoS Genet. 2014 May 1;10(5):e1004324. doi: 10.1371/journal.pgen.1004324. eCollection 2014 May. PLoS Genet. 2014. PMID: 24784154 Free PMC article.
References
- Bauer FF, Pretorius IS. Yeast stress response and fermentation efficiency: how to survive the making of wine – a review. S. Afr. J. Enol. 2000;21:27–51.
- Beardmore RE, Gudelj I, Lipson DA, Hurst LD. Metabolic trade-offs and the maintenance of the fittest and the flattest. Nature. 2011;472:342–346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases