Mechanics of dynamin-mediated membrane fission - PubMed (original) (raw)
Review
Mechanics of dynamin-mediated membrane fission
Sandrine Morlot et al. Annu Rev Biophys. 2013.
Abstract
In eukaryotic cells, membrane compartments are split into two by membrane fission. This ensures discontinuity of membrane containers and thus proper compartmentalization. The first proteic machinery implicated in catalyzing membrane fission was dynamin. Dynamin forms helical collars at the neck of endocytic buds. This structural feature suggested that the helix of dynamin could constrict in order to promote fission of the enclosed membrane. However, verifying this hypothesis revealed itself to be a challenge, which inspired many in vitro and in vivo studies. The primary goal of this review is to discuss recent structural and physical data from biophysical studies that have refined our understanding of the dynamin mechanism. In addition to the constriction hypothesis, other models have been proposed to explain how dynamin induces membrane fission. We present experimental data supporting these various models and assess which model is the most probable.
Figures
Figure 1
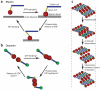
Dynamin assembly. (a) Schematic representations of dynamin helix in four different conditions: in solution, on membrane templates, on membrane templates with BAR domains, and in vivo. The helical diameter is identical in all conditions in the absence of GTP. However, the pitch is longer in the presence of BAR domains and in vivo. (b) Crystal (top) and schematic (bottom) structures of a dynamin dimer (PDB ID: 3SHN). The proline-rich domain (PRD) is not represented. Monomers interact in a cross shape via their stalk. (c) Schematic representations of a dynamin helix around a lipid tube ( gray). Radial (right) and transverse (left) views show how dimers assemble into a helix. Abbreviation: BSE, bundling signal element.
Figure 2
Force-generating cycles for myosin and dynamin. (a) Actomyosin ATPase cycle. Upon ATP binding the myosin head (red ) detaches from the actin filament ( gray). After ATP hydrolysis myosin rebinds to actin. Release of ADP triggers the powerstroke: mechanical motion of the lever arm. (b) Putative dynamin GTPase cycle. In the case of dynamin, the powerstroke occurs during GTP hydrolysis. According to structural data, conformational changes are observed between the GMP-PCP-bound state and the GDP AIF4−-bound state. Thus, GDP release could be linked to dissociation of the G domain from its substrate, · i.e., the opposite G domain in the consecutive turn. GTP binding could then reactivate the G domain interactions. (c) GTPase cycles for two consecutive rows of four dimers. After each monomer completes GTP hydrolysis, the two adjacent turns have walked on each other, thus constricting the lipid tube. Note that GTPase cycles are not synchronized. Here cycles of the eight dimers are represented concomitantly for clarity.
Figure 3
Constriction model. Membrane fission occurs in two steps. In the first step, the dynamin helix constricts the lipid nanotube so that its radius decreases. This step is controlled by the concentration of GTP, as GTP is the energy source of dynamin, and the torque subsequently delivered. In the second step, the constricted tube spontaneously hemifuses and breaks. The kinetics of this step depends on membrane elasticity.
Similar articles
- The long and short of membrane fission.
Roux A, Antonny B. Roux A, et al. Cell. 2008 Dec 26;135(7):1163-5. doi: 10.1016/j.cell.2008.12.003. Cell. 2008. PMID: 19109885 - Coarse-grained simulation of dynamin-mediated fission.
Fuhrmans M, Müller M. Fuhrmans M, et al. Soft Matter. 2015 Feb 28;11(8):1464-80. doi: 10.1039/c4sm02533d. Soft Matter. 2015. PMID: 25523542 - "Gearing" up for dynamin-catalyzed membrane fission.
Khurana H, Pucadyil TJ. Khurana H, et al. Curr Opin Cell Biol. 2023 Aug;83:102204. doi: 10.1016/j.ceb.2023.102204. Epub 2023 Jul 12. Curr Opin Cell Biol. 2023. PMID: 37451176 Review. - Membrane shape at the edge of the dynamin helix sets location and duration of the fission reaction.
Morlot S, Galli V, Klein M, Chiaruttini N, Manzi J, Humbert F, Dinis L, Lenz M, Cappello G, Roux A. Morlot S, et al. Cell. 2012 Oct 26;151(3):619-29. doi: 10.1016/j.cell.2012.09.017. Cell. 2012. PMID: 23101629 Free PMC article. - Dynamin: functional design of a membrane fission catalyst.
Schmid SL, Frolov VA. Schmid SL, et al. Annu Rev Cell Dev Biol. 2011;27:79-105. doi: 10.1146/annurev-cellbio-100109-104016. Epub 2011 May 18. Annu Rev Cell Dev Biol. 2011. PMID: 21599493 Review.
Cited by
- The tilted helix model of dynamin oligomers.
Kadosh A, Colom A, Yellin B, Roux A, Shemesh T. Kadosh A, et al. Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):12845-12850. doi: 10.1073/pnas.1903769116. Epub 2019 Jun 12. Proc Natl Acad Sci U S A. 2019. PMID: 31189604 Free PMC article. - Mechanisms of action of NME metastasis suppressors - a family affair.
Prunier C, Chavrier P, Boissan M. Prunier C, et al. Cancer Metastasis Rev. 2023 Dec;42(4):1155-1167. doi: 10.1007/s10555-023-10118-x. Epub 2023 Jun 24. Cancer Metastasis Rev. 2023. PMID: 37353690 Free PMC article. Review. - Dynamin 2 (DNM2) as Cause of, and Modifier for, Human Neuromuscular Disease.
Zhao M, Maani N, Dowling JJ. Zhao M, et al. Neurotherapeutics. 2018 Oct;15(4):966-975. doi: 10.1007/s13311-018-00686-0. Neurotherapeutics. 2018. PMID: 30426359 Free PMC article. Review. - Developmental and epileptic encephalopathies: from genetic heterogeneity to phenotypic continuum.
Guerrini R, Conti V, Mantegazza M, Balestrini S, Galanopoulou AS, Benfenati F. Guerrini R, et al. Physiol Rev. 2023 Jan 1;103(1):433-513. doi: 10.1152/physrev.00063.2021. Epub 2022 Aug 11. Physiol Rev. 2023. PMID: 35951482 Free PMC article. Review. - Cross-talk between the transcription factor Sp1 and C/EBPβ modulates TGFβ1 production to negatively regulate the expression of chemokine RANTES.
Sakamoto A, Yamaguchi R, Yamaguchi R, Narahara S, Sugiuchi H, Yamaguchi Y. Sakamoto A, et al. Heliyon. 2018 Jul 4;4(7):e00679. doi: 10.1016/j.heliyon.2018.e00679. eCollection 2018 Jul. Heliyon. 2018. PMID: 29998198 Free PMC article.
References
- Ahmadian MR, Hoffmann U, Goody RS, Wittinghofer A. Individual rate constants for the interaction of Ras proteins with GTPase-activating proteins determined by fluorescence spectroscopy. Biochemistry. 1997;36:4535–41. - PubMed
- Bagshaw CR. Muscle Contraction. Chapman & Hall; London/New York: 1993. p. 155.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources