The effects of chronic alcoholism on cell proliferation in the human brain - PubMed (original) (raw)
The effects of chronic alcoholism on cell proliferation in the human brain
G T Sutherland et al. Exp Neurol. 2013 Sep.
Abstract
Neurogenesis continues in the human subventricular zone and to a lesser extent in the hippocampal subgranular zone throughout life. Subventricular zone-derived neuroblasts migrate to the olfactory bulb where survivors become integrated as interneurons and are postulated to contribute to odor discrimination. Adult neurogenesis is dysregulated in many neurological, neurovascular and neurodegenerative diseases. Alcohol abuse can result in a neurodegenerative condition called alcohol-related brain damage. Alcohol-related brain damage manifests clinically as cognitive dysfunction and the loss of smell sensation (hyposmia) and pathologically as generalized white matter atrophy and focal neuronal loss. The exact mechanism linking chronic alcohol intoxication with alcohol-related brain damage remains largely unknown but rodent models suggest that decreased neurogenesis is an important component. We investigated this idea by comparing proliferative events in the subventricular zone and olfactory bulb of a well-characterized cohort of 15 chronic alcoholics and 16 age-matched controls. In contrast to the findings in animal models there was no difference in the number of proliferative cell nuclear antigen-positive cells in the subventricular zone of alcoholics (mean±SD=28.7±20.0) and controls (27.6±18.9, p=1.0). There were also no differences in either the total (p=0.89) or proliferative cells (p=0.98) in the granular cell layer of the olfactory bulb. Our findings show that chronic alcohol consumption does not affect cell proliferation in the human SVZ or olfactory bulb. In fact only microglial proliferation could be demonstrated in the latter. Therefore neurogenic deficits are unlikely to contribute to hyposmia in chronic alcoholics.
Keywords: Adult neurogenesis; Chronic alcoholism; Human brain tissue; Proliferation.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Fig. 1
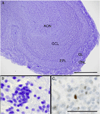
The anatomy and PCNA immunostaining of the adult human subventricular zone. This series of micrographs depicts the rostral region of the lateral aspect of the lateral ventricle wall in neurologically normal brains. (A) A photograph of a hemi-coronal section showing the corpus callosum (CC), the lateral ventricle (V) and the head of the caudate nucleus (CN). Scale bar = 1 cm. (B) A photomicrograph of a cresyl violet-stained thick section (48 µm) of the rostral lateral ventricle wall. The internal capsule (IC) is now more discernable than in (A) while black arrows mark the three sampling sites used in this study. Scale bar = 5 mm. (C–E) Cresyl violet-stained sections show the cytoarchitecture of the dorsal (C), middle (D) and ventral (E) regions of the lateral ventricle wall. Double-ended black arrows indicate the extent of the subventricular zone (SVZ) in each region. The SVZ itself and the hypocellular layer adjacent to the overlying ependymal layer (EP) increase in width in a dorso-ventral direction. In thick sections the EP appears to contain several layers, but this is a preparation artifact. (E inset) Luxol fast blue staining (with Fast Red counterstain) reveals the myelin layer (intense purple) that demarcates the SVZ from the underlying parenchyma. (F–H) Photomicrographs of PCNA immunostaining of the dorsal (F), middle (G) and ventral regions (H) of the ventricle wall. PCNA-positive cells in the SVZ are increased in a dorso-ventral direction whereas staining of the supposedly post-mitotic EP was more variable. Scale bars (C–H) = 100 µm.
Fig. 2
The anatomy and PCNA immunostaining of the adult human olfactory bulb. (A) A low power micrograph of a human OB shows its laminated structure. From the periphery, the OB is made up of the olfactory nerve layer (ONL), the glomerular layer (GL), the external plexiform layer (EPL) and the central granule cell layer (GCL). In this OB, the GCL surrounds a bulbar component of the diffuse anterior olfactory nucleus (AON). The mitral cell layer lies between the EPL and GCL. Scale bar = 0.5 mm. (B) A high power view of the GCL showing the typical clumping pattern of the micronuclear interneurons, the predominant cell type in this layer. (C) A single PCNA-positive cell is demonstrated within the GCL. Scale bars (B–C) = 50 µm.
Fig. 3
A comparison of Ki-67 and PCNA immunostaining in the adult human brain. These photomicrographs are of thin (7 µm) paraffin-embedded sections of a glioblastoma multiforme (GBM; WHO, grade 4) (A and D) and a more posterior (caudal) section of the lateral ventricle wall (adjacent to the nucleus accumbens) from a 64-year old male control (B, C, E, F). High power images of the GBM show equivalent amounts of (A) PCNA and (D) Ki-67 staining with a mitotic figure in the latter. Scale bars (A and D) = 50 µm. Low power images show the disparity between (B) PCNA and (E) Ki-67 staining in the lateral ventricle wall. A black arrow in (E) shows a single Ki-67 positive cell. In thin sections the ependymal layer is now seen as a single-layer cuboidal epithelium that is PCNA (B), but not Ki-67-positive. (E) Scale bars (B and E) = 200 µm. High power views of (C) PCNA and (F) Ki-67 immunostaining of the SVZ. Scale bars (C and F) = 100 µm.
Fig. 4
NeuN staining of the olfactory bulb. Positive immunostaining for the pan-neuronal marker NeuN was observed in short-term FFPE thin sections of the olfactory bulb from an additional neurologically normal individual (49-year old male). A low power micrograph from a section counterstained with haematoxylin (A) demonstrates the typical architecture of the human olfactory bulb including the densely packed granule cell layer (GCL). Other regions include the peripheral olfactory nerve layer (ONL), the glomerular layer (GL) with its distinctive non-cellular and oval-shaped glomeruli (black arrow) and the external plexiform layer (EPL) adjacent to the GCL. Scale bar = 500 µm. The high power micrograph (B) shows that the majority of the cells in the GCL are NeuN-positive interneurons. Scale bar = 100 µm.
Fig. 5
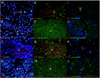
Co-localization studies in the granular cell layer. Immunofluorescence was used to determine the phenotype of the PCNA-positive cells in FFPE thin sections of the granule cell layer (GCL) from an additional neurologically normal individual (49-year old male). All sections were stained with the nuclear marker DAPI, although DAPI is not shown in images E–H for ease of viewing. The PCNA/DAPI (A–D) and corresponding PCNA/cell specific marker (E–H) widefield micrographs show rare PCNA-positive nuclei (white arrows). These widefield images show co-localization (yellow) between PCNA and the cytoplasmic microglial marker, Iba1 (E) but no co-localization with the nuclear mature neuronal marker, NeuN (G). There was also no obvious co-localization (yellow) between PCNA and the cytoplasmic markers for immature neurons, beta III tubulin (F), and astrocytes, GFAP (H). Confocal micrographs from areas adjacent to those used in the widefield images show co-localization of PCNA and Iba1 (I) but not beta III tubulin (J), NeuN (K) or GFAP (L). Scale bars = 100 µm (A–H) and 50 µm (I–L).
Similar articles
- Age-related impairment of olfactory bulb neurogenesis in the Ts65Dn mouse model of Down syndrome.
Bianchi P, Bettini S, Guidi S, Ciani E, Trazzi S, Stagni F, Ragazzi E, Franceschini V, Bartesaghi R. Bianchi P, et al. Exp Neurol. 2014 Jan;251:1-11. doi: 10.1016/j.expneurol.2013.10.018. Epub 2013 Nov 2. Exp Neurol. 2014. PMID: 24192151 - Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model.
Winner B, Desplats P, Hagl C, Klucken J, Aigner R, Ploetz S, Laemke J, Karl A, Aigner L, Masliah E, Buerger E, Winkler J. Winner B, et al. Exp Neurol. 2009 Oct;219(2):543-52. doi: 10.1016/j.expneurol.2009.07.013. Epub 2009 Jul 18. Exp Neurol. 2009. PMID: 19619535 Free PMC article. - Adult neurogenic process in the subventricular zone-olfactory bulb system is regulated by Tau protein under prolonged stress.
Dioli C, Patrício P, Pinto LG, Marie C, Morais M, Vyas S, Bessa JM, Pinto L, Sotiropoulos I. Dioli C, et al. Cell Prolif. 2021 Jul;54(7):e13027. doi: 10.1111/cpr.13027. Epub 2021 May 14. Cell Prolif. 2021. PMID: 33988263 Free PMC article. - Olfactory bulb neurogenesis depending on signaling in the subventricular zone.
Chen Y, Ren P, He X, Yan F, Gu R, Bai J, Zhang X. Chen Y, et al. Cereb Cortex. 2023 Nov 4;33(22):11102-11111. doi: 10.1093/cercor/bhad349. Cereb Cortex. 2023. PMID: 37746807 Review. - Neurogenesis and neuronal migration in the postnatal ventricular-subventricular zone: Similarities and dissimilarities between rodents and primates.
Akter M, Kaneko N, Sawamoto K. Akter M, et al. Neurosci Res. 2021 Jun;167:64-69. doi: 10.1016/j.neures.2020.06.001. Epub 2020 Jun 15. Neurosci Res. 2021. PMID: 32553727 Review.
Cited by
- Progenitor Cells Play a Role in Reinstatement of Ethanol Seeking in Adult Male and Female Ethanol Dependent Rats.
Nonoguchi HA, Jin M, Narreddy R, Kouo TWS, Nayak M, Trenet W, Mandyam CD. Nonoguchi HA, et al. Int J Mol Sci. 2023 Jul 31;24(15):12233. doi: 10.3390/ijms241512233. Int J Mol Sci. 2023. PMID: 37569609 Free PMC article. - Reduction of Cell Proliferation by Acute C2H6O Exposure.
Baldari S, Manni I, Di Rocco G, Paolini F, Palermo B, Piaggio G, Toietta G. Baldari S, et al. Cancers (Basel). 2021 Oct 5;13(19):4999. doi: 10.3390/cancers13194999. Cancers (Basel). 2021. PMID: 34638483 Free PMC article. - Effects of Cocaine on Human Glial-Derived Extracellular Vesicles.
Kumar S, Matthews QL, Sims B. Kumar S, et al. Front Cell Dev Biol. 2021 Jan 11;8:563441. doi: 10.3389/fcell.2020.563441. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33505956 Free PMC article. - Factors Associated With Phantom Odor Perception Among US Adults: Findings From the National Health and Nutrition Examination Survey.
Bainbridge KE, Byrd-Clark D, Leopold D. Bainbridge KE, et al. JAMA Otolaryngol Head Neck Surg. 2018 Sep 1;144(9):807-814. doi: 10.1001/jamaoto.2018.1446. JAMA Otolaryngol Head Neck Surg. 2018. PMID: 30128498 Free PMC article. - Molecular Neuropathology of Astrocytes and Oligodendrocytes in Alcohol Use Disorders.
Miguel-Hidalgo JJ. Miguel-Hidalgo JJ. Front Mol Neurosci. 2018 Mar 20;11:78. doi: 10.3389/fnmol.2018.00078. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29615864 Free PMC article. Review.
References
- Ahmed Z, Asi YT, Lees AJ, Revesz T, Holton JL. Identification and quantification of oligodendrocyte precursor cells in multiple system atrophy, progressive supranuclear palsy and Parkinson’s disease. Brain Pathol. 2012 http://dx.doi.org/10.1111/j.1750-3639.2012.00637.x (Article first published online: 23 OCT 2012) - DOI - PMC - PubMed
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969;137:433–457. - PubMed
- Alvarez I, Gonzalo LM, Llor J. Effects of chronic alcoholism on the amygdaloid complex. A study in human and rats. Histol. Histopathol. 1989;4:183–192. - PubMed
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: 2000. Text Revision.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical