Clonal development and organization of the adult Drosophila central brain - PubMed (original) (raw)
. 2013 Apr 22;23(8):633-43.
doi: 10.1016/j.cub.2013.02.057. Epub 2013 Mar 28.
Takeshi Awasaki, Mark David Schroeder, Fuhui Long, Jacob S Yang, Yisheng He, Peng Ding, Jui-Chun Kao, Gloria Yueh-Yi Wu, Hanchuan Peng, Gene Myers, Tzumin Lee
Affiliations
- PMID: 23541733
- PMCID: PMC3637848
- DOI: 10.1016/j.cub.2013.02.057
Clonal development and organization of the adult Drosophila central brain
Hung-Hsiang Yu et al. Curr Biol. 2013.
Abstract
Background: The insect brain can be divided into neuropils that are formed by neurites of both local and remote origin. The complexity of the interconnections obscures how these neuropils are established and interconnected through development. The Drosophila central brain develops from a fixed number of neuroblasts (NBs) that deposit neurons in regional clusters.
Results: By determining individual NB clones and pursuing their projections into specific neuropils, we unravel the regional development of the brain neural network. Exhaustive clonal analysis revealed 95 stereotyped neuronal lineages with characteristic cell-body locations and neurite trajectories. Most clones show complex projection patterns, but despite the complexity, neighboring clones often coinnervate the same local neuropil or neuropils and further target a restricted set of distant neuropils.
Conclusions: These observations argue for regional clonal development of both neuropils and neuropil connectivity throughout the Drosophila central brain.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Figure 1. Representative NB clones with stereotyped morphologies
(A-D) Merged confocal images of MARCM clones (green) in nc82-counterstained adult Drosophila brains (magenta). The same NB clone (SLPa&l1) was hit in [A] and [B]; a neighboring but distinct clone (LHl1) were identified in [C] and [D]. Note their possession of two versus one cluster of cell bodies (dashed circles) and the innervation of distinct sets of neuropils (arrows). (E-H) Adult SIPa1 (E, F) and VPNl&d1 (G, H) clones, induced shortly after larval hatching, were labeled with twin-spot MARCM. As revealed from the paired GMC clones (green), the first larval-born GMC yielded only one viable neuron in the SIPa1 lineage but produced two neurons in the VPNl&d1 lineage. Note the segregation of the twin VPNl&d1 neurons with distinct projections (H) into each of the two cell body clusters (dashed circles) present in the NB clone (G). (I-N) SMPad1, CREa2, and FLAa3 clones exhibit gender-specific neurite elaborations, as pointed out with arrows in the subpanels of [I] - [N]. (O-Q) PBp1, DM5, and DL1 clones show glia-like elaborations. Close-up views (insets) reveal astrocyte-like (as) glia in the PBp1 clone, both ensheathing (en) and astrocyte-like (as) glia in the DM5 clone, and optic lobe glia separating the medulla (ME) and lobule/lobule plate (LO/LOP) in the DL1 clone. Scale bars, 20 μm. Spatially segregated background clones were removed in some cases.
Figure 2. Catalog of cerebral NB clones
Ninety-two stereotyped NB clones (green) shown individually after warping into an nc82-counterstained adult Drosophila brain (magenta). They are named according to their primary immediate neuropil targets, referred to as home neuropils, and cataloged by grouping home neuropils into dorsal, lateral, anterior, and midline/posterior neuropil sets. Note the brains are shown with the anterior or posterior surface up depending on the location of the clone cell bodies. Spatially segregated background clones were removed in some cases. Note that the PSa1 clone originates from anterior brain surface. Please find the same images at higher resolution in supplemental Figure S2 and the raw 3D images in Virtual Fly Brain:
.
Figure 3. Cell body distribution of neuropil-characteristic neuronal lineages
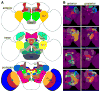
(A) Illustration of neuropils that have been arbitrarily assigned to three cross sections in the adult Drosophila brain. Anterior neuropils, AL: antennal lobe, AVLP: anterior ventrolateral protocerebrum, CRE: crepine, MB-LB: mushroom body lobe, PRW: prow. Inner neuropils, AOTU: anterior optic tubercle, BU: bulb, EB: ellipsoid body, FB: fan shaped body, FLA: flange, LAL: lateral accessory lobe, NO: Noduli, PVLP: posterior ventrolateral protocerebrum, SAD: saddle, SEG: subesophageal ganglion, SLP: superior lateral protocerebrum, SMP: superior medial protocerebrum, VES: vest, WED: wedge. Posterior neuropils, AME: accessory medulla, ATL: antler, CAN: cantle, EPA: epaulette, IB: inferior bridge, ICL: inferior clamp, IPS: inferior posterior slope, LH: lateral horn, LO: lobula, LOP: lobula plate, MB-CA: mushroom body calyx, ME: medulla, PB: protocerebral bridge, PLP: posterior lateral protocerebrum, SCL: superior clamp, SIP: superior intermediate protocerebrum, SPS: superior posterior slope. (B) Illustration of neuropil-characteristic clonal cell body distributions on the anterior or posterior brain surface. Clonal cell body loci for various neuropils shown in different colors in the top panels are superimposed in the bottom panel, to reveal the overall coverage by the identified clones.
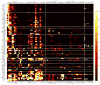
Figure 4. Heat map of neuropil innervations by various NB clones
Degrees of voxel coverage in distinct neuropils in the ipsilateral as well as contralateral hemisphere by 95 representative NB clones, including one female sample from each of the 92 stereotyped lineages plus three male clones showing obvious sexual dimorphism. Note the neurite elaborations in various optic lobe neuropils are simply indicated with white dots. Blue circles indicate home neuropils.
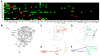
Figure 5. Putative clonal-level neuropil connectome
(A) The likely connections between various home neuropils (listed on the Y-axis) and other neuropil regions (arranged along the X-axis) are indicated with green boxes. This putative neuropil connectome was deduced primarily through the determination of the major distal targets shared by most of the NB clones cofounding a home neuropil, as revealed from the heat map of clonal neuropil innervation patterns (Figure 4). For those home neuropils founded by no more than two lineages (*), their possible distal targets were identified by manually tracking the readily traceable neurite fascicles in individual clones. The reciprocal connections between the SMP and FLA (#) are evident in the relatively simple FLPa3, and SMPpv1 clones; the AL-to-MB-CA connection (#) is known. (B) Diagrams of possible information flow across distinct neuropils in the Drosophila cerebrum, as judged from the neuropil connection matrix shown in [A]. And neural activities presumably propagate from home neuropils to their connected neuropils, except that the EB lies distal to the BU in the EBa1 clone, the sole EB founding lineage. The putative sub-networks that process olfactory or visual information are illustrated in additional diagrams.
Similar articles
- Systematic analysis of neural projections reveals clonal composition of the Drosophila brain.
Ito M, Masuda N, Shinomiya K, Endo K, Ito K. Ito M, et al. Curr Biol. 2013 Apr 22;23(8):644-55. doi: 10.1016/j.cub.2013.03.015. Epub 2013 Mar 28. Curr Biol. 2013. PMID: 23541729 - Neural lineages of the Drosophila brain: a three-dimensional digital atlas of the pattern of lineage location and projection at the late larval stage.
Pereanu W, Hartenstein V. Pereanu W, et al. J Neurosci. 2006 May 17;26(20):5534-53. doi: 10.1523/JNEUROSCI.4708-05.2006. J Neurosci. 2006. PMID: 16707805 Free PMC article. - Generation and Evolution of Neural Cell Types and Circuits: Insights from the Drosophila Visual System.
Perry M, Konstantinides N, Pinto-Teixeira F, Desplan C. Perry M, et al. Annu Rev Genet. 2017 Nov 27;51:501-527. doi: 10.1146/annurev-genet-120215-035312. Epub 2017 Sep 27. Annu Rev Genet. 2017. PMID: 28961025 Free PMC article. Review. - Lineages to circuits: the developmental and evolutionary architecture of information channels into the central complex.
Kandimalla P, Omoto JJ, Hong EJ, Hartenstein V. Kandimalla P, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2023 Jul;209(4):679-720. doi: 10.1007/s00359-023-01616-y. Epub 2023 Mar 17. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2023. PMID: 36932234 Free PMC article. Review.
Cited by
- Stem cell-specific ecdysone signaling regulates the development of dorsal fan-shaped body neurons and sleep homeostasis.
Wani AR, Chowdhury B, Luong J, Chaya GM, Patel K, Isaacman-Beck J, Kayser MS, Syed MH. Wani AR, et al. Curr Biol. 2024 Nov 4;34(21):4951-4967.e5. doi: 10.1016/j.cub.2024.09.020. Epub 2024 Oct 8. Curr Biol. 2024. PMID: 39383867 - Whole-brain annotation and multi-connectome cell typing of Drosophila.
Schlegel P, Yin Y, Bates AS, Dorkenwald S, Eichler K, Brooks P, Han DS, Gkantia M, Dos Santos M, Munnelly EJ, Badalamente G, Serratosa Capdevila L, Sane VA, Fragniere AMC, Kiassat L, Pleijzier MW, Stürner T, Tamimi IFM, Dunne CR, Salgarella I, Javier A, Fang S, Perlman E, Kazimiers T, Jagannathan SR, Matsliah A, Sterling AR, Yu SC, McKellar CE; FlyWire Consortium; Costa M, Seung HS, Murthy M, Hartenstein V, Bock DD, Jefferis GSXE. Schlegel P, et al. Nature. 2024 Oct;634(8032):139-152. doi: 10.1038/s41586-024-07686-5. Epub 2024 Oct 2. Nature. 2024. PMID: 39358521 Free PMC article. - The Hox Gene, abdominal A controls timely mitotic entry of neural stem cell and their growth during CNS development in Drosophila.
Das P, Murthy S, Abbas E, White K, Arya R. Das P, et al. bioRxiv [Preprint]. 2024 Sep 5:2024.09.04.611161. doi: 10.1101/2024.09.04.611161. bioRxiv. 2024. PMID: 39282366 Free PMC article. Preprint. - Translational regulation enhances distinction of cell types in the nervous system.
Ichinose T, Kondo S, Kanno M, Shichino Y, Mito M, Iwasaki S, Tanimoto H. Ichinose T, et al. Elife. 2024 Jul 16;12:RP90713. doi: 10.7554/eLife.90713. Elife. 2024. PMID: 39010741 Free PMC article. - Neurotransmitter classification from electron microscopy images at synaptic sites in Drosophila melanogaster.
Eckstein N, Bates AS, Champion A, Du M, Yin Y, Schlegel P, Lu AK, Rymer T, Finley-May S, Paterson T, Parekh R, Dorkenwald S, Matsliah A, Yu SC, McKellar C, Sterling A, Eichler K, Costa M, Seung S, Murthy M, Hartenstein V, Jefferis GSXE, Funke J. Eckstein N, et al. Cell. 2024 May 9;187(10):2574-2594.e23. doi: 10.1016/j.cell.2024.03.016. Cell. 2024. PMID: 38729112 Free PMC article.
References
- Stocker RF, Lienhard MC, Borst A, Fischbach KF. Neuronal architecture of the antennal lobe in Drosophila melanogaster. Cell Tissue Res. 1990;262:9–34. - PubMed
- Leiss F, Groh C, Butcher NJ, Meinertzhagen IA, Tavosanis G. Synaptic organization in the adult Drosophila mushroom body calyx. J Comp Neurol. 2009;517:808–824. - PubMed
- Young JM, Armstrong JD. Structure of the adult central complex in Drosophila: organization of distinct neuronal subsets. J Comp Neurol. 2010;518:1500–1524. - PubMed
- Rein K, Zockler M, Mader MT, Grubel C, Heisenberg M. The Drosophila standard brain. Curr Biol. 2002;12:227–231. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous