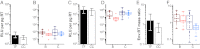Phenotypic properties of transmitted founder HIV-1 - PubMed (original) (raw)
Comparative Study
. 2013 Apr 23;110(17):6626-33.
doi: 10.1073/pnas.1304288110. Epub 2013 Mar 29.
Feng Gao, Hui Li, Elena E Giorgi, Hannah J Barbian, Erica H Parrish, Lara Zajic, Shilpa S Iyer, Julie M Decker, Amit Kumar, Bhavna Hora, Anna Berg, Fangping Cai, Jennifer Hopper, Thomas N Denny, Haitao Ding, Christina Ochsenbauer, John C Kappes, Rachel P Galimidi, Anthony P West Jr, Pamela J Bjorkman, Craig B Wilen, Robert W Doms, Meagan O'Brien, Nina Bhardwaj, Persephone Borrow, Barton F Haynes, Mark Muldoon, James P Theiler, Bette Korber, George M Shaw, Beatrice H Hahn
Affiliations
- PMID: 23542380
- PMCID: PMC3637789
- DOI: 10.1073/pnas.1304288110
Comparative Study
Phenotypic properties of transmitted founder HIV-1
Nicholas F Parrish et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Defining the virus-host interactions responsible for HIV-1 transmission, including the phenotypic requirements of viruses capable of establishing de novo infections, could be important for AIDS vaccine development. Previous analyses have failed to identify phenotypic properties other than chemokine receptor 5 (CCR5) and CD4+ T-cell tropism that are preferentially associated with viral transmission. However, most of these studies were limited to examining envelope (Env) function in the context of pseudoviruses. Here, we generated infectious molecular clones of transmitted founder (TF; n = 27) and chronic control (CC; n = 14) viruses of subtypes B (n = 18) and C (n = 23) and compared their phenotypic properties in assays specifically designed to probe the earliest stages of HIV-1 infection. We found that TF virions were 1.7-fold more infectious (P = 0.049) and contained 1.9-fold more Env per particle (P = 0.048) compared with CC viruses. TF viruses were also captured by monocyte-derived dendritic cells 1.7-fold more efficiently (P = 0.035) and more readily transferred to CD4+ T cells (P = 0.025). In primary CD4+ T cells, TF and CC viruses replicated with comparable kinetics; however, when propagated in the presence of IFN-α, TF viruses replicated to higher titers than CC viruses. This difference was significant for subtype B (P = 0.000013) but not subtype C (P = 0.53) viruses, possibly reflecting demographic differences of the respective patient cohorts. Together, these data indicate that TF viruses are enriched for higher Env content, enhanced cell-free infectivity, improved dendritic cell interaction, and relative IFN-α resistance. These viral properties, which likely act in concert, should be considered in the development and testing of AIDS vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Virion infectivity and Env content. (A–D) Infectivity values for TF and CC viruses (x axis) are expressed as relative light units (RLUs) per picogram of viral RT activity (y axis). (A) Bars indicate the median infectivity of TF (filled) and CC (open) viruses, with interquartile ranges indicated. TF viruses were 1.7-fold more infectious than CC viruses (P = 0.049). (B) Infectivity values are shown for each virus. Subtypes B and C viruses are shown in red and blue, respectively, with TF viruses indicated in dark colors and CC viruses indicated in light colors, respectively. Values represent averages from four independent experiments. (C and D) Infectivity values are shown for TF and CC viruses as in A and B, except that infections were performed in the presence of DEAE dextran. Values represent averages from three independent experiments. (E and F) Env content of TF and CC virions (x axis) is expressed as the mass ratio of Env and RT content (y axis). (E) Bars indicate the median values of Env content for TF (filled) and CC (open) viruses, with interquartile ranges indicated. TF viruses contained 1.9 times more Env per unit of RT activity than CC viruses (P = 0.048). (F) Env content is shown for each virus and color-coded as in B and D. Values represent averages from two independent experiments.
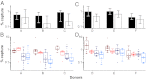
Fig. 2.
Virus binding to moDCs. The percent of captured TF and CC virus is plotted (y axis) for moDC cell preparations from six different donors labeled A through F (x axis). (A and B) Virus input was normalized by RT activity. (C and D) Virus input was normalized by p24 content. (A and C) Bars indicate median values of moDC capture for TF (filled) and CC (open) viruses, with interquartile ranges indicated. TF viruses were captured 1.7-fold more efficiently than CC viruses (P = 0.035). (B and D) Values are plotted for each virus individually (color-coding for TF and CC viruses from subtypes B and C as in Fig. 1). Subtype B viruses were captured 3.4 times more efficiently than subtype C viruses (P = 4.6 × 10−6 by GLM).
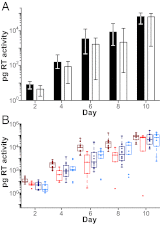
Fig. 3.
DC-mediated trans infection. (A) Virus replication expressed as picograms of RT activity per milliliter of culture supernatant (y axis) is shown after cocultivation of virus-pulsed moDC with CD4+ T cells for one representative donor (of two analyzed) over 10 d (x axis). Bars indicate median values of viral replication for TF (filled) and CC (open) viruses, with interquartile ranges indicated (there was no significant difference between TF and CC viruses). (B) Values are plotted for each virus individually (color-coding for TF and CC viruses from subtypes B and C as in Fig. 1). Averaging data from two different donors, subtype B TF viruses grew to 11.2-fold higher titers than subtype B CC viruses (P = 0.004), whereas no significant differences were observed for subtype C TF and CC viruses (P = 0.23).
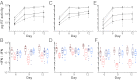
Fig. 4.
Virus replication in CD4+ T cells in the presence and absence of IFN-α. (A, C, and E) The replication kinetics of TF (solid lines) and CC (broken lines) viruses are shown in CD4+ T cells from three donors in the absence (gray lines) and presence (black lines) of 500 U IFN-α. RT activity indicated as picograms per milliliter of culture supernatant (y axis) was measured every 3 d (x axis). Data points indicate median values of virus production, with interquartile ranges indicated. Averaging data from all donors and points, TF viruses grew to 24-fold higher titers than CC viruses in the presence of IFN-α (P = 0.012). (B, D, and F) The ratio of virus production in the presence and absence of IFN-α is plotted for each virus (y axis) at different time points (days) postinfection (x axis; color-coding for TF and CC viruses from subtypes B and C as in Fig. 1). Averaging data from all donors, subtype B TF viruses grew to 62-fold higher titers than subtype B CC viruses (P = 0.000013), whereas subtype C TF viruses grew only 1.7-fold more efficiently than subtype C CC viruses (P = 0.53). Note that these replication differentials represent cumulative totals for the 12-d culture period.
Similar articles
- Characterization of the Plasmacytoid Dendritic Cell Response to Transmitted/Founder and Nontransmitted Variants of HIV-1.
Schwartz JA, Zhang H, Ende Z, Deymier MJ, Lee T, Singer J, Mazzulli T, Hunter E, Ostrowski MA. Schwartz JA, et al. J Virol. 2018 Sep 12;92(19):e00157-18. doi: 10.1128/JVI.00157-18. Print 2018 Oct 1. J Virol. 2018. PMID: 29997203 Free PMC article. - Unique Phenotypic Characteristics of Recently Transmitted HIV-1 Subtype C Envelope Glycoprotein gp120: Use of CXCR6 Coreceptor by Transmitted Founder Viruses.
Ashokkumar M, Aralaguppe SG, Tripathy SP, Hanna LE, Neogi U. Ashokkumar M, et al. J Virol. 2018 Apr 13;92(9):e00063-18. doi: 10.1128/JVI.00063-18. Print 2018 May 1. J Virol. 2018. PMID: 29491151 Free PMC article. - Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7.
Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, Salazar MG, Decker JM, Parrish EH, Berg A, Hopper J, Hora B, Kumar A, Mahlokozera T, Yuan S, Coleman C, Vermeulen M, Ding H, Ochsenbauer C, Tilton JC, Permar SR, Kappes JC, Betts MR, Busch MP, Gao F, Montefiori D, Haynes BF, Shaw GM, Hahn BH, Doms RW. Parrish NF, et al. PLoS Pathog. 2012;8(5):e1002686. doi: 10.1371/journal.ppat.1002686. Epub 2012 May 31. PLoS Pathog. 2012. PMID: 22693444 Free PMC article. - Differential utilization of CD4+ by transmitted/founder and chronic envelope glycoproteins in a MSM HIV-1 subtype B transmission cluster.
Bouvin-Pley M, Leoz M, Roch E, Moreau A, Migraine J, Bellini N, Blake O, Mammano F, Braibant M, Plantier JC, Brand D. Bouvin-Pley M, et al. AIDS. 2020 Dec 1;34(15):2187-2200. doi: 10.1097/QAD.0000000000002690. AIDS. 2020. PMID: 32932339 - HIV-1 envelope, integrins and co-receptor use in mucosal transmission of HIV.
Cicala C, Arthos J, Fauci AS. Cicala C, et al. J Transl Med. 2011 Jan 27;9 Suppl 1(Suppl 1):S2. doi: 10.1186/1479-5876-9-S1-S2. J Transl Med. 2011. PMID: 21284901 Free PMC article. Review.
Cited by
- Host cell glycosylation selects for infection with CCR5- versus CXCR4-tropic HIV-1.
Itell HL, Guenthoer J, Humes D, Baumgarten NE, Overbaugh J. Itell HL, et al. Nat Microbiol. 2024 Nov;9(11):2985-2996. doi: 10.1038/s41564-024-01806-7. Epub 2024 Oct 3. Nat Microbiol. 2024. PMID: 39363105 - Mosaic HIV-1 vaccine and SHIV challenge strain V2 loop sequence identity and protection in primates.
Vanshylla K, Tolboom J, Stephenson KE, Feddes-de Boer K, Verwilligen A, Rosendahl Huber SK, Rutten L, Schuitemaker H, Zahn RC, Barouch DH, Wegmann F. Vanshylla K, et al. NPJ Vaccines. 2024 Sep 30;9(1):179. doi: 10.1038/s41541-024-00974-1. NPJ Vaccines. 2024. PMID: 39349488 Free PMC article. - The combination of three CD4-induced antibodies targeting highly conserved Env regions with a small CD4-mimetic achieves potent ADCC activity.
Marchitto L, Richard J, Prévost J, Tauzin A, Yang D, Chiu T-J, Chen H-C, Díaz-Salinas MA, Nayrac M, Benlarbi M, Beaudoin-Bussières G, Anand SP, Dionne K, Bélanger É, Chatterjee D, Medjahed H, Bourassa C, Tolbert WD, Hahn BH, Munro JB, Pazgier M, Smith AB 3rd, Finzi A. Marchitto L, et al. J Virol. 2024 Oct 22;98(10):e0101624. doi: 10.1128/jvi.01016-24. Epub 2024 Sep 9. J Virol. 2024. PMID: 39248460 - Help or Hinder: Protein Host Factors That Impact HIV-1 Replication.
Moezpoor MR, Stevenson M. Moezpoor MR, et al. Viruses. 2024 Aug 10;16(8):1281. doi: 10.3390/v16081281. Viruses. 2024. PMID: 39205255 Free PMC article. Review. - Conformational flexibility of HIV-1 envelope glycoproteins modulates transmitted/founder sensitivity to broadly neutralizing antibodies.
Parthasarathy D, Pothula KR, Ratnapriya S, Cervera Benet H, Parsons R, Huang X, Sammour S, Janowska K, Harris M, Sodroski J, Acharya P, Herschhorn A. Parthasarathy D, et al. Nat Commun. 2024 Aug 26;15(1):7334. doi: 10.1038/s41467-024-51656-4. Nat Commun. 2024. PMID: 39187497 Free PMC article.
References
- Zhang Z, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286(5443):1353–1357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI027767/AI/NIAID NIH HHS/United States
- P30 AI27767/AI/NIAID NIH HHS/United States
- UM1 AI100645/AI/NIAID NIH HHS/United States
- T32 AI007632/AI/NIAID NIH HHS/United States
- R01 AI045378/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- T32 AI07632/AI/NIAID NIH HHS/United States
- U19 AI067854/AI/NIAID NIH HHS/United States
- R37 AI045378/AI/NIAID NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- R01 AI04088/AI/NIAID NIH HHS/United States
- K08 AI093153/AI/NIAID NIH HHS/United States
- R01 AI45378/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- P30 AI45008/AI/NIAID NIH HHS/United States
- MR/K012037/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous