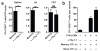Sensing and alarm function of resident memory CD8⁺ T cells - PubMed (original) (raw)
Sensing and alarm function of resident memory CD8⁺ T cells
Jason M Schenkel et al. Nat Immunol. 2013 May.
Erratum in
- Nat Immunol. 2013 Aug;14(8):876
Abstract
CD8(+) T cells eliminate intracellular infections through two contact-dependent effector functions: cytolysis and secretion of antiviral cytokines. Here we identify the following additional function for memory CD8(+) T cells that persist at front-line sites of microbial exposure: to serve as local sensors of previously encountered antigens that precipitate innate-like alarm signals and draw circulating memory CD8(+) T cells into the tissue. When memory CD8(+) T cells residing in the female mouse reproductive tract encountered cognate antigen, they expressed interferon-γ (IFN-γ), potentiated robust local expression of inflammatory chemokines and induced rapid recruitment of circulating memory CD8(+) T cells. Anamnestic responses in front-line tissues are thus an integrated collaboration between front-line and circulating populations of memory CD8(+) T cells, and vaccines should establish both populations to maximize rapid responses.
Figures
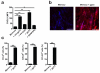
Figure 1. Local antigen re-challenge precipitates rapid accumulation of antigen-specific CD8+ T cells within the female reproductive tract (FRT)
Widely disseminated Thy1.1+ memory P14 CD8+ T cells were established after LCMV infection. Immune mice were challenged transcervically (t.c.) with 4×105 p.f.u. VV-gp33 or VV-OVA, 50μg gp33 or SIINFEKL peptide, or left untreated. (a) 48 h later, P14 cells in FRT were enumerated relative to total nucleated cells. (b) Representative images show α-Thy1.1 (red) and DAPI (blue) staining. Scale bars = 100 μm. n = 6, one representative of three experiments. n = 6, pooled from 2 experiments. *, P < 0.05, **, P <0.001, unpaired two-tailed _t_-test, mean±SEM.
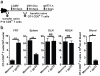
Figure 2. Local antigen re-challenge precipitates rapid inflammatory chemokine expression within the female reproductive tract (FRT)
Widely disseminated Thy1.1+ memory P14 CD8+ T cells were established after LCMV infection. Immune mice were challenged transcervically (t.c.) with 4×105 p.f.u. VV-gp33 or VV-OVA, 50μg gp33 or SIINFEKL peptide, or left untreated. (a) 48 h later, mean fluorescence intensity (MFI) of CXCL9 expression on CD31+ vessels was determined 12 h after t.c. challenge. (b) Representative images show α-CXCL9 (red) and α-CD31 (blue). Scale bars = 100 μm. (c) 12 h after t.c. gp33 challenge, CCL2, CXCL9 and CCL3/4 expression was evaluated on the indicated cell populations by flow cytometry. (a,b) n = 6, one representative of three experiments. (c) n = 6, pooled from 2 experiments. *, P < 0.05, **, P < 0.001, unpaired two-tailed _t_-test, mean±SEM.
Figure 3. Unstimulated memory CD8+ T cells redistribute when other memory CD8+ T cells are reactivated
(a) Two populations of memory CD8+ T cells with different specificities, Thy1.1+ P14 cells (gp33-specific) and CD45.1+ OT-I cells (SIINFEKL-specific), were established. (b) Mice were challenged with gp33. 48 h later, OT-I cells were enumerated in the FRT, spleen, blood, draining lymph node (DLN) and non-draining lymph node (NDLN). n ≥ 5, *, P < 0.05, **, P < 0.001, unpaired two-tailed _t_-test, mean±SEM.
Figure 4. Memory CD8+ T cells within nonlymphoid tissue orchestrate rapid recruitment of unstimulated memory, but not naïve, CD8+ T cells to the site of antigen re-exposure
(a) Thy1.1+ memory P14 cells were depleted from recirculating compartments but preserved in the FRT via administration of complement fixing α-Thy1.1 Ab. P14 cells were evaluated in tissues 72 h after Ab treatment. (b) Naïve mice, P14 immune chimeras, and P14 immune chimeras depleted of circulating P14 cells received i.v. transfers of naïve or memory OT-I CD8+ T cells, as indicated. 24 h after OT-I transfer, mice were challenged t.c. with gp33 and transferred OT-I cells were enumerated 48 h later within 7 μm coronal sections through the entire FRT. n = 3, representative of 2-3 independent experiments. *, P < 0.01, **, P <0.001, unpaired two-tailed _t_-test, mean±SEM.
Figure 5. IFN-γ and resident memory CD8+ T cells are required for rapid recruitment of memory T cells to the site of antigen reexposure
(a) OT-I immune chimeras were challenged t.c. with SIINFEKL. 12 h later, OT-I cells and IFN-γ were visualized by immunofluorescence microscopy. (b) OT-I.Ifng+/+ and OT-I._Ifng_−/− immune chimeras were challenged t.c. with SIINFEKL. 48 h later, CD8β+ cells were enumerated in FRT. (c) CD45.1+ memory OT-I cells were transferred to naïve recipients, which were then conjoined to Thy1.1+ P14 immune chimeric mice via parabiosis. 14-16 days after surgery, memory P14 cells were enumerated in both conjoined parabionts. (d) Parabionts (as in c) were challenged with gp33 t.c. and 48 h later OT-Is were quantified within 7 μm coronal sections through the entire FRT. Scale bars = 50 μm.. Data pooled from two independent experiments totaling 5-6 mice per group. *, P <0.001, unpaired two-tailed _t_-test, mean±SEM.
Similar articles
- Simultaneous assessment of antigen-stimulated cytokine production and memory subset composition of memory CD8 T cells.
Jabbari A, Harty JT. Jabbari A, et al. J Immunol Methods. 2006 Jun 30;313(1-2):161-8. doi: 10.1016/j.jim.2006.04.005. Epub 2006 May 26. J Immunol Methods. 2006. PMID: 16762359 - Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections.
Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Brehm MA, et al. J Immunol. 2003 Apr 15;170(8):4077-86. doi: 10.4049/jimmunol.170.8.4077. J Immunol. 2003. PMID: 12682237 - CD8alphaalpha-mediated survival and differentiation of CD8 memory T cell precursors.
Madakamutil LT, Christen U, Lena CJ, Wang-Zhu Y, Attinger A, Sundarrajan M, Ellmeier W, von Herrath MG, Jensen P, Littman DR, Cheroutre H. Madakamutil LT, et al. Science. 2004 Apr 23;304(5670):590-3. doi: 10.1126/science.1092316. Science. 2004. PMID: 15105501 - Mice deficient in stem cell antigen-1 (Sca1, Ly-6A/E) develop normal primary and memory CD4+ and CD8+ T-cell responses to virus infection.
Whitmire JK, Eam B, Whitton JL. Whitmire JK, et al. Eur J Immunol. 2009 Jun;39(6):1494-504. doi: 10.1002/eji.200838959. Eur J Immunol. 2009. PMID: 19384870 Free PMC article. - Evolution of the CD8 T-cell repertoire during infections.
Lin MY, Selin LK, Welsh RM. Lin MY, et al. Microbes Infect. 2000 Jul;2(9):1025-39. doi: 10.1016/s1286-4579(00)01257-0. Microbes Infect. 2000. PMID: 10967283 Review.
Cited by
- CD4+ T cells persist for years in the human small intestine and display a TH1 cytokine profile.
Bartolomé-Casado R, Landsverk OJB, Chauhan SK, Sætre F, Hagen KT, Yaqub S, Øyen O, Horneland R, Aandahl EM, Aabakken L, Bækkevold ES, Jahnsen FL. Bartolomé-Casado R, et al. Mucosal Immunol. 2021 Mar;14(2):402-410. doi: 10.1038/s41385-020-0315-5. Epub 2020 Jun 22. Mucosal Immunol. 2021. PMID: 32572129 - Emerging concepts in tissue-resident T cells: lessons from humans.
Thome JJ, Farber DL. Thome JJ, et al. Trends Immunol. 2015 Jul;36(7):428-35. doi: 10.1016/j.it.2015.05.003. Epub 2015 Jun 10. Trends Immunol. 2015. PMID: 26072286 Free PMC article. Review. - Innate lymphoid cell function in the context of adaptive immunity.
Bando JK, Colonna M. Bando JK, et al. Nat Immunol. 2016 Jun 21;17(7):783-9. doi: 10.1038/ni.3484. Nat Immunol. 2016. PMID: 27328008 Free PMC article. - Tumor-specific memory CD8+ T cells are strictly resident in draining lymph nodes during tumorigenesis.
Liu Q, Ran L, Yue Z, Su X, Wang L, Wen S, Lei S, Yang X, Zhang Y, Hu J, Tang J, Li Z, Hu L, Zhu B, Xu L, Ye L, Huang Q. Liu Q, et al. Cell Mol Immunol. 2023 Apr;20(4):423-426. doi: 10.1038/s41423-023-00986-2. Epub 2023 Mar 1. Cell Mol Immunol. 2023. PMID: 36859455 Free PMC article. No abstract available. - Migration and Function of Memory CD8+ T Cells in Skin.
Hirai T, Whitley SK, Kaplan DH. Hirai T, et al. J Invest Dermatol. 2020 Apr;140(4):748-755. doi: 10.1016/j.jid.2019.09.014. Epub 2019 Dec 4. J Invest Dermatol. 2020. PMID: 31812277 Free PMC article. Review.
References
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. - PubMed
- Andrian, von UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. - PubMed
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. - PubMed
- Akira S, Uematsu S. Pathogen recognition and innate immunity. Cell. 2006;240:783–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI084913/AI/NIAID NIH HHS/United States
- T32 AI007313/AI/NIAID NIH HHS/United States
- R01 AI084913/AI/NIAID NIH HHS/United States
- DP2 OD006467/OD/NIH HHS/United States
- R01AI084913-01/AI/NIAID NIH HHS/United States
- DP2OD006467/OD/NIH HHS/United States
- T32AI007313/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials