Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes - PubMed (original) (raw)
Randomized Controlled Trial
. 2013 Sep;62(9):3224-31.
doi: 10.2337/db12-1512. Epub 2013 Apr 1.
Affiliations
- PMID: 23545707
- PMCID: PMC3749332
- DOI: 10.2337/db12-1512
Randomized Controlled Trial
Effect of losartan on prevention and progression of early diabetic nephropathy in American Indians with type 2 diabetes
E Jennifer Weil et al. Diabetes. 2013 Sep.
Erratum in
- Erratum. Effect of Losartan on Prevention and Progression of Early Diabetic Nephropathy in American Indians With Type 2 Diabetes. Diabetes 2013;62:3224-3231.
Weil EJ, Fufaa G, Jones LI, Lovato T, Lemley KV, Hanson RL, Knowler WC, Bennett PH, Yee B, Myers BD, Nelson RG. Weil EJ, et al. Diabetes. 2018 Mar;67(3):532. doi: 10.2337/db18-er03a. Epub 2018 Jan 5. Diabetes. 2018. PMID: 29305527 Free PMC article. No abstract available.
Abstract
Angiotensin receptor blockers are renoprotective in hypertensive azotemic patients with type 2 diabetes, but their efficacy in early diabetic kidney disease is uncertain. We performed a 6-year randomized clinical trial in 169 American Indians with type 2 diabetes and normoalbuminuria (albumin/creatinine ratio [ACR] <30 mg/g; n = 91) or microalbuminuria (ACR 30-299 mg/g; n = 78) at baseline. The primary outcome was decline in glomerular filtration rate (GFR) to ≤60 mL/min or to half the baseline value in subjects who entered with GFR <120 mL/min. Another outcome was differences in glomerular structure at end of treatment. Subjects received 100 mg losartan or placebo daily. GFR was measured annually; 111 subjects underwent kidney biopsies. Only nine subjects reached the GFR outcome, and the unadjusted hazard ratio (losartan vs. placebo) was 0.50 (95% CI, 0.12-1.99). Differences in mesangial fractional volume were not estimated in the combined albuminuria groups because of an interaction with treatment assignment. In separate analyses, mesangial fractional volume was lower in subjects treated with losartan in the microalbuminuria group (18.8 vs. 25.6%; P = 0.02), but not in the normoalbuminuria group (19.6 vs. 17.8%; P = 0.86). Treatment with losartan may preserve some features of kidney structure in American Indians with type 2 diabetes and microalbuminuria.
Figures
FIG. 1.
Enrollment, randomization, and follow-up of the study subjects. All subjects were included in intention-to-treat analyses, except one person in the normoalbuminuria group who was randomized to losartan but could not be found after the baseline examination.
FIG. 2.
Annual mean ± standard error MAP (upper panels), HbA1c (middle panels), and GFR (lower panels) in subjects with normoalbuminuria (left panels) or microalbuminuria (right panels) at baseline by treatment group (dashed line = placebo; solid line = losartan). There were no significant differences in any of these measures by treatment group throughout the treatment period (for MAP, P = 0.21 in the normoalbuminuria group and P = 0.73 in the microalbuminuria group; for HbA1c, P = 0.48 in the normoalbuminuria group and P = 0.43 in the microalbuminuria group; and for GFR, P = 0.11 in the normoalbuminuria group and P = 0.42 in the microalbuminuria group).
FIG. 3.
Cumulative percentage of the first occurrence of the GFR outcome by treatment group (dashed line = placebo; solid line = losartan). Log-rank tests for the GFR outcome yielded P = 0.31.
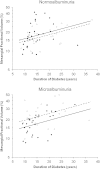
FIG. 4.
Effect of treatment with losartan or placebo on mesangial fractional volume (logarithmic scale) according to duration of diabetes in subjects with normoalbuminuria or microalbuminuria at baseline (open circles [○] = treatment with placebo; filled circles [●] = treatment with losartan). Regression lines are shown by treatment group (dashed line = placebo; solid line = losartan). After adjusting for duration of diabetes, mesangial fractional volume was 22.9% (95% CI, 4.6–37.7%) lower in subjects with microalbuminuria who received losartan than in those who received placebo. Mesangial fractional volume was not statistically significantly higher (10.1% [−8.9 to 33.0%]) in subjects with normoalbuminuria who received losartan than in those who received placebo.
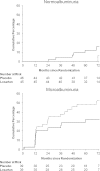
FIG. 5.
Cumulative percentage of the first occurrence of the macroalbuminuria outcome in subjects with normoalbuminuria or microalbuminuria at baseline by treatment group (dashed line = placebo; solid line = losartan). Log-rank tests for the macroalbuminuria outcome yielded P = 0.02 in the normoalbuminuria group and P = 0.08 in the microalbuminuria group.
Comment in
- Does losartan prevent progression of early diabetic nephropathy in American Indians with type 2 diabetes?
Nicholas SB, Iyengar SK. Nicholas SB, et al. Diabetes. 2013 Sep;62(9):3014-6. doi: 10.2337/db13-0748. Diabetes. 2013. PMID: 23970520 Free PMC article. No abstract available.
References
- Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 - PubMed
- Lewis EJ, Hunsicker LG, Clarke WR, et al. Collaborative Study Group . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 - PubMed
- Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2009;54:205–226 - PubMed
- Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870–878 - PubMed
- Gaede P, Vedel P, Larsen N, Jensen GVH, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical