IFNβ/TNFα synergism induces a non-canonical STAT2/IRF9-dependent pathway triggering a novel DUOX2 NADPH oxidase-mediated airway antiviral response - PubMed (original) (raw)
IFNβ/TNFα synergism induces a non-canonical STAT2/IRF9-dependent pathway triggering a novel DUOX2 NADPH oxidase-mediated airway antiviral response
Karin Fink et al. Cell Res. 2013 May.
Erratum in
- Cell Res. 2014 Apr;24(4):509
Abstract
Airway epithelial cells are key initial innate immune responders in the fight against respiratory viruses, primarily via the secretion of antiviral and proinflammatory cytokines that act in an autocrine/paracrine fashion to trigger the establishment of an antiviral state. It is currently thought that the early antiviral state in airway epithelial cells primarily relies on IFNβ secretion and the subsequent activation of the interferon-stimulated gene factor 3 (ISGF3) transcription factor complex, composed of STAT1, STAT2 and IRF9, which regulates the expression of a panoply of interferon-stimulated genes encoding proteins with antiviral activities. However, the specific pathways engaged by the synergistic action of different cytokines during viral infections, and the resulting physiological outcomes are still ill-defined. Here, we unveil a novel delayed antiviral response in the airways, which is initiated by the synergistic autocrine/paracrine action of IFNβ and TNFα, and signals through a non-canonical STAT2- and IRF9-dependent, but STAT1-independent cascade. This pathway ultimately leads to the late induction of the DUOX2 NADPH oxidase expression. Importantly, our study uncovers that the development of the antiviral state relies on DUOX2-dependent H2O2 production. Key antiviral pathways are often targeted by evasion strategies evolved by various pathogenic viruses. In this regard, the importance of the novel DUOX2-dependent antiviral pathway is further underlined by the observation that the human respiratory syncytial virus is able to subvert DUOX2 induction.
Figures
Figure 1
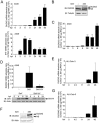
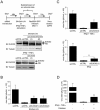
DUOX2 and DUOXA2 are induced upon SeV infection in AECs. (A–C) A549 cells were infected with SeV (40 HAU/106 cells) for the indicated times. (D) A549 cells were infected with SeV or UV-treated SeV (40 HAU/106 cells) for the indicated times. (E–G) Polarized Calu-3 cells cultured for 10 days in ALI (ALI-Calu-3) and presenting an UAR ≥ 800 Ω.cm2 were infected with SeV (40 HAU/106 cells) at the apical side for the indicated times. In A, C, D, E and G, total RNA was extracted. DUOX2, IFIT1 or DUOXA2 mRNA absolute copy numbers were quantified by qRT-PCR. In B and F, DUOX2 protein expression was analyzed by immunoblot analyses using anti-DUOX1/2-specific antibodies. In D, SeV N protein expression was detected using anti-parainfluenza antibodies. Equal loading was verified using anti-tubulin or anti-actin antibody. All data are presented as mean ± SD. Statistical analyses were conducted using one-way ANOVA with Tukey post-test, except in D, where analysis was performed using a _t_-test. Statistical significances are presented compared with the non-infected control, except in D, where the SeV-infected condition is compared with SeV-UV-infected condition. *P < 0.05, ***P < 0.001. The dotted line in qRT-PCR graphs represents the threshold of detection. IB, immunoblot; NS, non specific; hpi, hours post-infection; HAU, hemagglutinin units; UAR, unit area resistance.
Figure 2
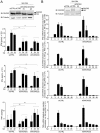
SeV induces DUOX2 and DUOXA2 expression through secreted proteins. (A) A schematic outline of the timeline used for experiments in B–F. (B–D) A549 cells were stimulated with untreated (SN-SeV) or UV-treated supernatants (SN-SeV-UV) from SeV-infected (40 HAU/106 cells) for 24 h (C, D) or the indicated times (B). SeV infection (40 HAU/106 cells, 24 h) was conducted as comparison. (E) A549 cells were stimulated with SN-SeV-UV or SN-SeV-UV subjected to heat treatment. In B, D and E, DUOX2 or DUOXA2 mRNA expression was analyzed by qRT-PCR. In B, C, and E, immunoblot analyses were performed to measure the protein expression levels of phosphorylated STAT1 (STAT1-P-Tyr701), STAT1, SeV, DUOX2 or IFIT1. (F) A549 cells were treated for 24 h with SN-SeV-UV generated from cells exposed to DMSO (SN-SeV-UV/DMSO) or 0.1 mM Z-VAD-FMK (SN-SeV-UV/Z-VAD-FMK). DUOX2 mRNA expression was analyzed by qRT-PCR. PARP cleavage was assessed in SeV-infected A549 cells treated with DMSO or Z-VAD-FMK, as well as in cells stimulated with SN-SeV-UV/DMSO or SN-SeV-UV/Z-VAD-FMK. qRT-PCR data are presented as mean ± SD. Statistical analysis was conducted using one-way ANOVA with Dunnett post-test, except in E and F, where a _t_-test was used. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3
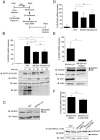
Costimulation by IFNβ and TNFα efficiently induces DUOX2 and DUOXA2 expression and DUOX2-dependent H2O2 production. (A, B) A549 cells were stimulated with recombinant IFNβ and/or TNFα for 24 h. SeV infection (40 HAU/106 cells, 24 h) was conducted for comparison. DUOX2 or DUOXA2 mRNA absolute copy numbers were analyzed by qRT-PCR. In A, DUOX2 protein expression was analyzed by immunoblot analyses. (C) A549 cells were infected with SeV (40 HAU/106 cells). IFNβ and TNFα levels in the supernatants were measured by Multiplex ELISA. (D) A549 cells were transfected with control siRNA (siCTRL) or siRNA targeting IFNAR1 using a mixture of siIFNAR1(1) and siIFNAR1(2), and/or siRNA targeting TNFRSF1A using siTNFRSF1A. Forty-eight hours post-transfection, cells were stimulated with SN-SeV-UV for 24 h. IFNAR1 expression levels were analyzed by qRT-PCR and TNFRI levels were detected by immunoblot analyses. (E) A549 cells were stimulated as in A. Where indicated, the cells were transfected with siCTRL or siRNA targeting DUOX2 (siDUOX2(1)) 48 h prior to stimulation. H2O2 production was analyzed using the HVA assay. Where indicated, catalase was added at 400 U/ml. All qRT-PCR and H2O2 measurement data are presented as mean ± SD. Fold induction is calculated over the corresponding non-stimulated condition. The pointed line in graphs represents the threshold of detection. Statistical analysis was conducted by one-way ANOVA using Tukey multiple comparison analysis. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4
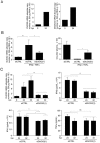
DUOX2 induction is regulated in a STAT2/IRF9-dependent, STAT1-independent manner. (A–C) A549 cells were transfected with siRNA specific for STAT1, STAT2 or IRF9. Forty eight h post-transfection, cells were stimulated with SN-SeV-UV for 24 h in A and B or IFNγ for the indicated time in C. (D) A549 cells were pretreated with AG490 (100 μM), Bayer-18 (100 μM) or DMSO (vehicle) for 1 h before stimulation with IFNβ and TNFα for 24 h. In A, B and D, DUOX2 mRNA absolute copy number was analyzed by qRT-PCR and DUOX2 levels were expressed as % of the siCTRL condition (A, B) or as % of the control cells (D). In A–E, DUOX2, STAT2-P-Tyr690, STAT2, STAT1-P-Tyr701, STAT1, IκBα-P-Ser32, IκBα, or IRF9 protein levels were analyzed by immunoblot. All qRT-PCR data are presented as mean ± SD. Data were analyzed by one-way ANOVA with Dunnett post-test. *P < 0.05, *** P < 0.001.
Figure 5
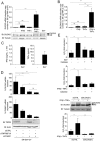
DUOX2 is necessary for the establishment of an H2O2-dependent antiviral state. (A) A schematic outline of the experimental timeline used for experiments in B–D. (B–D) A549 cells were transfected with siCTRL, siDUOX2(1) or siDUOX2(2) before being stimulated with SN-SeV-UV or with IFNβ and TNFα for 24 h. DUOX2 expression was analyzed by immunoblot analyses using DUOX1/2 antibody. Twenty-four h post-stimulation with SN-SeV-UV or cytokines, cells were infected with recombinant RecRSV-GFP at a MOI of 1 for 72 h and the release of infectious viral particles was quantified by plaque forming unit assay. Catalase was added 6 h prior to RecRSV-GFP infection in D. All data are presented as mean ± SD. Data were analyzed by one-way ANOVA with Dunnett post-test, siCTRL vs siDUOX2(1) or siDUOX2(2) in B and C, or IFNβ + TNFα vs IFNβ + TNFα + catalase in D. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 6
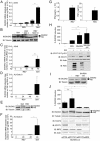
DUOX2 regulates secreted levels of type I/III IFNs at the late stages of viral infection. (A) A549 cells were transfected with siCTRL, siDUOX2(1) or siDUOX2(2), and 48 h post-transfection, cells were infected with SeV (40 HAU/106 cells) for the indicated times. DUOX2 expression was analyzed by immunoblot analyses using DUOX1/2 specific antibodies. Release of IFNβ, IFNλ and TNFα was measured by multiplex ELISA. (B) A549 cells were transfected with siCTRL or siDUOX2(2) for 48 h and infected as in A for the indicated times. IFNβ, IFNλ (IL-28A and IL-29) and TNFα mRNA absolute copy numbers were analyzed by qRT-PCR. Data were analyzed by two-way ANOVA with Bonferroni post-test. *P < 0.05, **P < 0.01, ***P < 0.001. The dotted line in qRT-PCR graphs represents the threshold of detection.
Figure 7
DUOX2 induction controls a late IFNβ/TNFα-dependent antiviral state in NHBEs. (A) NHBEs were infected with SeV (40 HAU/106 cells) for 24 h. DUOX2 or DUOXA2 expression levels were analyzed by qRT-PCR. (B, C) NHBEs were transfected with siCTRL or siDUOX2(1) and then stimulated with IFNβ and TNFα for 24h before RecRSV-GFP infection (B), or infected with SeV for the indicated times (C). DUOX2 expression levels were analyzed by qRT-PCR. In B, infectious virion release was quantified as described in Figure 5. In C, release of IFNβ, IFNλ and TNFα was measured by multiplex ELISA. Data were analyzed by one-way ANOVA with Dunnett post-test in B. Data were analyzed by two-way ANOVA with Bonferroni post-test in C. *P < 0.05, **P < 0.01, ***P < 0.001. The dotted line in qRT-PCR graphs represents the threshold of detection.
Figure 8
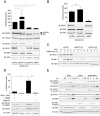
SN-RSV-UV triggers higher expression of DUOX2 than direct RSV infection. (A, C) A549 cells were infected with RSV at an MOI of 3 for the indicated times. SeV infection (40 HAU/106 cells) was conducted for comparison. (B) A549 cells were infected with RSV at an MOI of 3 or 10 or with SeV at 40 HAU/106 cells for 24 h. (D–F) ALI-Calu-3 cells were infected with RSV at an MOI of 3 for the indicated times, or for 24h in E. (G) A549 cells were infected with RSV at an MOI of 3 for 24 h. IFNβ/TNFα levels in the supernatants were measured by multiplex ELISA. (H, I) A549 cells were stimulated with SN-RSV or UV-treated supernatants (SN-RSV-UV) for 24 h. RSV infection (MOI = 3, 24 h) was conducted for comparison. (J) A549 cells were transfected with siRNAs as in Figure 4A, and 48 h post-transfection, cells were stimulated with SN-RSV-UV for 24 h. In A, C, D, F, H, and J, DUOX2 or DUOXA2 mRNA levels were quantified by qRT-PCR. These values are presented as % DUOX2 induction in H and J. In B, E, H, I and J, immunoblot analyses were performed to analyze the protein expression of DUOX2, STAT1-P-Tyr701, STAT1, RSV, STAT2 or IRF9. All qRT-PCR data are presented as mean ± SD. Data were analyzed by one-way ANOVA with Tukey post-test except in J, where a Dunnett post-test was used; *P < 0.05, **P < 0.01, ***P < 0.001. The pointed line in qRT-PCR quantification data represents the threshold of detection.
Figure 9
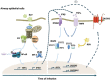
Model of the innate immune antiviral response triggered by IFNβ and TNFα in AECs. SeV infection of AECs triggers the secretion of IFNβ and TNFα. Binding of IFNβ to its cognate receptor activates the “classic” antiviral pathway mediated by the ISGF3 TF. Additionally, the synergism between IFNβ and TNFα induces late DUOX2 expression through a non-canonical antiviral signaling pathway. This pathway involves STAT2 and IRF9, but is entirely independent of STAT1. Late DUOX2 induction and H2O2 production is essential for the cells to mount an efficient antiviral state, at least in part through the regulation of IFNβ and IFNλ levels at late time points of infection. The importance of this novel airway antiviral defense mechanism is underlined by the observation that pathogenic RSV is able to counteract DUOX2 induction, suggesting that RSV has evolved a strategy to evade the DUOX2-dependent antiviral response.
Similar articles
- The Combination of IFN β and TNF Induces an Antiviral and Immunoregulatory Program via Non-Canonical Pathways Involving STAT2 and IRF9.
Mariani MK, Dasmeh P, Fortin A, Caron E, Kalamujic M, Harrison AN, Hotea DI, Kasumba DM, Cervantes-Ortiz SL, Mukawera E, Serohijos AWR, Grandvaux N. Mariani MK, et al. Cells. 2019 Aug 17;8(8):919. doi: 10.3390/cells8080919. Cells. 2019. PMID: 31426476 Free PMC article. - STAT2/IRF9 directs a prolonged ISGF3-like transcriptional response and antiviral activity in the absence of STAT1.
Blaszczyk K, Olejnik A, Nowicka H, Ozgyin L, Chen YL, Chmielewski S, Kostyrko K, Wesoly J, Balint BL, Lee CK, Bluyssen HA. Blaszczyk K, et al. Biochem J. 2015 Mar 15;466(3):511-24. doi: 10.1042/BJ20140644. Biochem J. 2015. PMID: 25564224 Free PMC article. - Unphosphorylated ISGF3 drives constitutive expression of interferon-stimulated genes to protect against viral infections.
Wang W, Yin Y, Xu L, Su J, Huang F, Wang Y, Boor PPC, Chen K, Wang W, Cao W, Zhou X, Liu P, van der Laan LJW, Kwekkeboom J, Peppelenbosch MP, Pan Q. Wang W, et al. Sci Signal. 2017 Apr 25;10(476):eaah4248. doi: 10.1126/scisignal.aah4248. Sci Signal. 2017. PMID: 28442624 - The unique role of STAT2 in constitutive and IFN-induced transcription and antiviral responses.
Blaszczyk K, Nowicka H, Kostyrko K, Antonczyk A, Wesoly J, Bluyssen HA. Blaszczyk K, et al. Cytokine Growth Factor Rev. 2016 Jun;29:71-81. doi: 10.1016/j.cytogfr.2016.02.010. Epub 2016 Mar 18. Cytokine Growth Factor Rev. 2016. PMID: 27053489 Review. - The emerging role of interferon regulatory factor 9 in the antiviral host response and beyond.
Suprunenko T, Hofer MJ. Suprunenko T, et al. Cytokine Growth Factor Rev. 2016 Jun;29:35-43. doi: 10.1016/j.cytogfr.2016.03.002. Epub 2016 Mar 4. Cytokine Growth Factor Rev. 2016. PMID: 26987614 Review.
Cited by
- Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases.
Nauseef WM. Nauseef WM. Biochim Biophys Acta. 2014 Feb;1840(2):757-67. doi: 10.1016/j.bbagen.2013.04.040. Epub 2013 May 7. Biochim Biophys Acta. 2014. PMID: 23660153 Free PMC article. Review. - Overexpressing IFITM family genes predict poor prognosis in kidney renal clear cell carcinoma.
Xu Y, Huang D, Zhang K, Tang Z, Ma J, Zhu M, Xiong H. Xu Y, et al. Transl Androl Urol. 2021 Oct;10(10):3837-3851. doi: 10.21037/tau-21-848. Transl Androl Urol. 2021. PMID: 34804826 Free PMC article. - Host metabolic reprogramming in response to SARS-CoV-2 infection: A systems biology approach.
Moolamalla STR, Balasubramanian R, Chauhan R, Priyakumar UD, Vinod PK. Moolamalla STR, et al. Microb Pathog. 2021 Sep;158:105114. doi: 10.1016/j.micpath.2021.105114. Epub 2021 Jul 30. Microb Pathog. 2021. PMID: 34333072 Free PMC article. - System-wide identification of myeloid markers of TB disease and HIV-induced reactivation in the macaque model of Mtb infection and Mtb/SIV co-infection.
Gough M, Singh DK, Singh B, Kaushal D, Mehra S. Gough M, et al. Front Immunol. 2022 Oct 5;13:777733. doi: 10.3389/fimmu.2022.777733. eCollection 2022. Front Immunol. 2022. PMID: 36275677 Free PMC article. - Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses.
Hristova M, Habibovic A, Veith C, Janssen-Heininger YM, Dixon AE, Geiszt M, van der Vliet A. Hristova M, et al. J Allergy Clin Immunol. 2016 May;137(5):1545-1556.e11. doi: 10.1016/j.jaci.2015.10.003. Epub 2015 Nov 17. J Allergy Clin Immunol. 2016. PMID: 26597162 Free PMC article.
References
- Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010;31:278–287. - PubMed
- Harper RW, Xu C, Soucek K, Setiadi H, Eiserich JP. A reappraisal of the genomic organization of human Nox1 and its splice variants. Arch Biochem Biophys. 2005;435:323–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous