Genomic copy number alterations in clear cell renal carcinoma: associations with case characteristics and mechanisms of VHL gene inactivation - PubMed (original) (raw)
doi: 10.1038/oncsis.2012.14.
E Jaeger, M L Nickerson, P Brennan, S De Vries, R Roy, J Toro, H Li, S Karami, P Lenz, D Zaridze, V Janout, V Bencko, M Navratilova, N Szeszenia-Dabrowska, D Mates, W M Linehan, M Merino, J Simko, R Pfeiffer, P Boffetta, S Hewitt, N Rothman, W-H Chow, F M Waldman
Affiliations
- PMID: 23552698
- PMCID: PMC3412648
- DOI: 10.1038/oncsis.2012.14
Genomic copy number alterations in clear cell renal carcinoma: associations with case characteristics and mechanisms of VHL gene inactivation
L E Moore et al. Oncogenesis. 2012.
Abstract
Array comparative genomic hybridization was used to identify copy number alterations in clear cell renal cell carcinoma (ccRCC) patient tumors to identify associations with patient/clinical characteristics. Of 763 ccRCC patients, 412 (54%) provided frozen biopsies. Clones were analyzed for significant copy number differences, adjusting for multiple comparisons and covariates in multivariate analyses. Frequent alterations included losses on: 3p (92.2%), 14q (46.8%), 8p (38.1%), 4q (35.4%), 9p (32.3%), 9q (31.8%), 6q (30.8%), 3q (29.4%), 10q (25.7%), 13q (24.5%), 1p (23.5%) and gains on 5q (60.2%), 7q (39.6%), 7p (30.6%), 5p (26.5%), 20q (25.5%), 12q (24.8%), 12p (22.8%). Stage and grade were associated with 1p, 9p, 9q, 13q and 14q loss and 12q gain. Males had more alterations compared with females, independent of stage and grade. Significant differences in the number/types of alterations were observed by family cancer history, age at diagnosis and smoking status. Von Hippel-Lindau (VHL) gene inactivation was associated with 3p loss (P<E-05), and these cases had fewer alterations than wild-type cases. The fragile site flanking the FHIT locus (3p14.2) represented a unique breakpoint among VHL hypermethylated cases, compared with wild-type cases and those with sequence changes. This is the first study of its size to investigate copy number alterations among cases with extensive patient, clinical/risk factor information. Patients characterized by VHL wild-type gene status (vs sequence alterations) and male (vs female) cases had more copy number alterations regardless of diagnostic stage and grade, which could relate to poor prognosis.
Figures
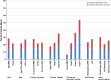
Figure 1
Fraction (%) of the FGL, FGG and FGA among ccRCC case subgroups. _P_-values between subgroups were as follows: males vs females (_P_=0.002), age (<50, vs ⩾50 years, _P_=0.06), any family history of cancer (_P_=0.02), stage (P<0.00001), grade (P<0.0001), ever vs never smoking (_P_=0.05), VHL wild-type cases vs those with VHL promoter hypermethylation (_P_=0.03), VHL wild-type cases vs those with VHL sequence alterations (_P_=0.17). REF, referent group.
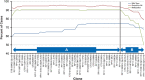
Figure 2
Frequency of copy number loss of 3p clones among ccRCC cases grouped by absence (wild-type) or presence of VHL gene inactivation (through sequence alteration or promoter hypermethylation). Region A (telomere to RP11-180G14): the frequency of copy number loss of individual 3p clones differed significantly between VHL wild-type and inactivated cases that occurred through both sequence alteration (P<0.00001) and promoter hypermethylation (_P_=0.03–0.001). Region B (from RP11-180G14to RP11-154H23): the frequency of clonal copy number loss differed significantly between wild-type cases and those with sequence alterations (_P_=0.003–<0.00001), but was no longer observed with hypermethylated cases (_P_=0.08–0.59).
Similar articles
- Patterns of gene expression and copy-number alterations in von-hippel lindau disease-associated and sporadic clear cell carcinoma of the kidney.
Beroukhim R, Brunet JP, Di Napoli A, Mertz KD, Seeley A, Pires MM, Linhart D, Worrell RA, Moch H, Rubin MA, Sellers WR, Meyerson M, Linehan WM, Kaelin WG Jr, Signoretti S. Beroukhim R, et al. Cancer Res. 2009 Jun 1;69(11):4674-81. doi: 10.1158/0008-5472.CAN-09-0146. Cancer Res. 2009. PMID: 19470766 Free PMC article. - Specific genomic aberrations predict survival, but low mutation rate in cancer hot spots, in clear cell renal cell carcinoma.
Köhn L, Svenson U, Ljungberg B, Roos G. Köhn L, et al. Appl Immunohistochem Mol Morphol. 2015 May-Jun;23(5):334-42. doi: 10.1097/PAI.0000000000000087. Appl Immunohistochem Mol Morphol. 2015. PMID: 24992170 Free PMC article. - Genomic aberrations in carcinomas of the uterine corpus.
Micci F, Teixeira MR, Haugom L, Kristensen G, Abeler VM, Heim S. Micci F, et al. Genes Chromosomes Cancer. 2004 Jul;40(3):229-46. doi: 10.1002/gcc.20038. Genes Chromosomes Cancer. 2004. PMID: 15139002 - Cytogenetic aberrations in primary and recurrent fibrolamellar hepatocellular carcinoma detected by comparative genomic hybridization.
Wilkens L, Bredt M, Flemming P, Kubicka S, Klempnauer J, Kreipe H. Wilkens L, et al. Am J Clin Pathol. 2000 Dec;114(6):867-74. doi: 10.1309/BMTT-JBPD-D13H-1UVD. Am J Clin Pathol. 2000. PMID: 11338475 - Genetic and epigenetic alterations during renal carcinogenesis.
Arai E, Kanai Y. Arai E, et al. Int J Clin Exp Pathol. 2010 Dec 13;4(1):58-73. Int J Clin Exp Pathol. 2010. PMID: 21228928 Free PMC article. Review.
Cited by
- Stratification of clear cell renal cell carcinoma (ccRCC) genomes by gene-directed copy number alteration (CNA) analysis.
Thiesen HJ, Steinbeck F, Maruschke M, Koczan D, Ziems B, Hakenberg OW. Thiesen HJ, et al. PLoS One. 2017 May 9;12(5):e0176659. doi: 10.1371/journal.pone.0176659. eCollection 2017. PLoS One. 2017. PMID: 28486536 Free PMC article. - Novel drugs that target the metabolic reprogramming in renal cell cancer.
van der Mijn JC, Panka DJ, Geissler AK, Verheul HM, Mier JW. van der Mijn JC, et al. Cancer Metab. 2016 Jul 13;4:14. doi: 10.1186/s40170-016-0154-8. eCollection 2016. Cancer Metab. 2016. PMID: 27418963 Free PMC article. Review. - Immune cell infiltration signatures identified molecular subtypes and underlying mechanisms in gastric cancer.
Lin Y, Pan X, Zhao L, Yang C, Zhang Z, Wang B, Gao Z, Jiang K, Ye Y, Wang S, Shen Z. Lin Y, et al. NPJ Genom Med. 2021 Oct 11;6(1):83. doi: 10.1038/s41525-021-00249-x. NPJ Genom Med. 2021. PMID: 34635662 Free PMC article. - Current Landscape of Genomic Biomarkers in Clear Cell Renal Cell Carcinoma.
Cotta BH, Choueiri TK, Cieslik M, Ghatalia P, Mehra R, Morgan TM, Palapattu GS, Shuch B, Vaishampayan U, Van Allen E, Ari Hakimi A, Salami SS. Cotta BH, et al. Eur Urol. 2023 Aug;84(2):166-175. doi: 10.1016/j.eururo.2023.04.003. Epub 2023 Apr 19. Eur Urol. 2023. PMID: 37085424 Free PMC article. Review. - Monosomy of Chromosome 9 Is Associated With Higher Grade, Advanced Stage, and Adverse Outcome in Clear-cell Renal Cell Carcinoma.
Nejati R, Wei S, Uzzo RG, Poureghbali S, Pei J, Talarchek JN, Ruth K, Dulaimi E, Kutikov A, Testa JR, Al-Saleem T. Nejati R, et al. Clin Genitourin Cancer. 2020 Feb;18(1):56-61. doi: 10.1016/j.clgc.2019.09.016. Epub 2019 Sep 26. Clin Genitourin Cancer. 2020. PMID: 31648964 Free PMC article.
References
- Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Meanue M, et al. (2008)Cancer Incidence in Five Continents vol. IXIARC Scientific Publications; Lyon, France, 1980.
- Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163–2172. - PubMed
- Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor-suppressor gene. Science. 1993;260:1317–1320. - PubMed
- Maxwell PH, Weisener MS, Chang GW, Clifford SC, Vaux EC, Cockman M, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. - PubMed