The N and C termini of ZO-1 are surrounded by distinct proteins and functional protein networks - PubMed (original) (raw)
The N and C termini of ZO-1 are surrounded by distinct proteins and functional protein networks
Christina M Van Itallie et al. J Biol Chem. 2013.
Abstract
Background: Biotin ligase tagging with ZO-1 was applied to identify a more complete tight junction proteome.
Results: Identical but also different proteins and functional networks were identified near the N and C ends of ZO-1.
Conclusion: The ends of ZO-1 are embedded in different functional subcompartments of the tight junction.
Significance: Biotin tagging with ZO-1 expands the tight junction proteome and defines subcompartments of the junction. The proteins and functional protein networks of the tight junction remain incompletely defined. Among the currently known proteins are barrier-forming proteins like occludin and the claudin family; scaffolding proteins like ZO-1; and some cytoskeletal, signaling, and cell polarity proteins. To define a more complete list of proteins and infer their functional implications, we identified the proteins that are within molecular dimensions of ZO-1 by fusing biotin ligase to either its N or C terminus, expressing these fusion proteins in Madin-Darby canine kidney epithelial cells, and purifying and identifying the resulting biotinylated proteins by mass spectrometry. Of a predicted proteome of ∼9000, we identified more than 400 proteins tagged by biotin ligase fused to ZO-1, with both identical and distinct proteins near the N- and C-terminal ends. Those proximal to the N terminus were enriched in transmembrane tight junction proteins, and those proximal to the C terminus were enriched in cytoskeletal proteins. We also identified many unexpected but easily rationalized proteins and verified partial colocalization of three of these proteins with ZO-1 as examples. In addition, functional networks of interacting proteins were tagged, such as the basolateral but not apical polarity network. These results provide a rich inventory of proteins and potential novel insights into functions and protein networks that should catalyze further understanding of tight junction biology. Unexpectedly, the technique demonstrates high spatial resolution, which could be generally applied to defining other subcellular protein compartmentalization.
Keywords: Adherens Junction; Mass Spectrometry (MS); Membrane; Proteomics; Tight Junctions; ZO-1.
Figures
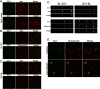
FIGURE 1.
ZO-1 biotin ligase fusion proteins but not biotin ligase alone colocalize with endogenous ZO-1. A, neither biotin ligase alone (myc, top middle) nor biotinylated proteins in the same cells (Streptavidin, bottom middle) colocalize (Merge, right panels) with endogenous ZO-1 (left panels) in MDCK cells. In contrast, both biotin ligase fused to the N terminus (B, BL-ZO1, myc) and C terminus (C, ZO1-BL) of ZO-1 colocalized (Merge, B and C, top right) with endogenous ZO-1 (B and C, top left panels; the monoclonal antibody to ZO-1 recognizes the canine but not the human protein). In addition, the majority of biotinylated proteins (Streptavidin, B and C, bottom middle panels) were also colocalized (B and C, bottom right) with endogenous ZO-1 (B and C, bottom left). D (left panels), confocal _z_-sections reveal that both BL-ZO-1 (myc) and biotinylated proteins (Streptavidin) are concentrated (Merge) with endogenous ZO-1 (left). D (right panels), similarly, ZO-1-BL (myc) and biotinylated proteins are also concentrated (Merge) with ZO-1 (right), although there is also some non-junctional immunofluorescence associated with both the transgene and the biotinylated proteins. E, MDCK cells expressing biotin ligase-ZO-1 were cultured in normal (top panels) or low calcium (bottom panels) medium supplemented with 50 μ
m
biotin for 15 h. Immunofluorescent localization of transgene (Myc) and biotinylated proteins (Streptavidin) revealed that incubation in low calcium resulted in the loss of most of the tight junction-associated protein, although remnants remain (arrows); some Myc and streptavidin signal appears vesicular (arrowheads), whereas most is diffuse. Bar, 20 μm.
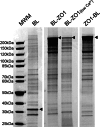
FIGURE 2.
Coomassie-stained SDS-PAGE reveals that streptavidin-purified biotinylated proteins from MDCK cells expressing different transgenes show differing protein patterns. Shown are proteins purified from biotin-treated cells expressing biotin ligase alone (BL), biotin ligase fused to the N terminus of ZO-1 (BL-ZO1), cells expressing the same construct but incubated in low calcium medium overnight (BL-ZO1, low Ca 2+), and cells expressing biotin ligase fused to the C terminus of ZO-1 (ZO1-BL). The positions of the transgenes are marked with arrowheads; the protein pattern for BL-ZO-1 in normal, and low calcium is quite similar. Triplicate samples gave very similar protein patterns. MWM, molecular weight markers.
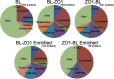
FIGURE 3.
Functional analysis of proteins recovered from MDCK cells expressing biotin ligase alone and ZO-1 biotin ligase fusion proteins. Top, streptavidin-purified proteins identified by mass spectrometry from cells expressing biotin ligase alone (left chart) or biotin ligase fused to the N terminus (Biotin Ligase-ZO1, middle) or C terminus of ZO-1 (ZO1-Biotin Ligase, right). Functional classification revealed similar distribution for the two ZO-1 constructs, whereas the largest fraction of proteins tagged from cells expressing biotin ligase alone were classified as “other,” including proteins involved in DNA and RNA synthesis, metabolism, etc. TJ+AJ, tight junction and adherens junction proteins. Bottom, in contrast, functional analysis of proteins recovered exclusively or >3-fold enriched (as determined by the averaged normalized PSMs) from ZO-1-BL-expressing (left) and BL-ZO-1-expressing (right) cells reveals differences in several categories, including tight junction and adherens junction, signaling, polarity, and other membrane proteins and kinases and phosphatases. For the purposes of this analysis, DVL1 and DVL3 were included with polarity proteins because of their interactions with VANG1, VANG2, and MARK2.
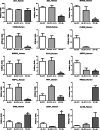
FIGURE 4.
Comparison of the relative amounts of specific proteins recovered from cells expressing biotin ligase fused to the N-terminal end of ZO-1 incubated in normal (BL-ZO1) or low calcium (BL-ZO1, lo Ca) or to the C-terminal end of ZO-1 (ZO1-BL). ZO-1 was recovered equally from all samples (top left), whereas differing amounts of other proteins were recovered from MDCK cells treated with 50 μ
m
biotin for 15 h and expressing the different constructs. Normalized PSMs were calculated as described under “Experimental Procedures”; n = 3 for each bar, mean ± S.E (error bars). *, p < 0.05 by analysis of variance followed by Dunnett's test.
FIGURE 5.
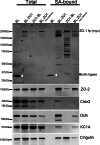
Immunoblot of selected proteins purified on streptavidin resin from MDCK cells expressing biotin ligase alone (BL), biotin ligase fused to the N-terminal (BL-ZO1) or C-terminal end of ZO-1 (ZO1-BL), or BL-ZO-1-expressing cells incubated in low calcium. Shown are samples from cell lysate before (left side, total) and after purification on streptavidin resin (right side, SA-bound). Cells were probed for the fusion protein with an anti-Myc antibody (top panel; white arrowheads mark the location of the biotin ligase alone fusion protein); the same samples were also probed for ZO-2 (second panel), claudin-2 (third panel), occludin (Ocln; fourth panel), CK1A (fifth panel), and cingulin (bottom panel).
FIGURE 6.
Confocal microscopy reveals that several proteins identified by biotinylation as ZO-1 neighbors partially colocalize with ZO-1 in MDCK cells. Top panels, endogenous FBP1L (TOCA-1; middle panel) is localized both intracellularly and at cell contacts. ZO-1 immunofluorescence (left panel) demarcates the tight junction; FBP1L staining overlaps with ZO-1 signal at the tight junction (right panel, Merge; green, ZO-1; red, FBP1L). Middle panels, similarly, ZO-1 (left panel) and GFP-EFR3A (middle panel) are partially colocalized (Merge, right panel; red, ZO-1; green, GFP), although GFP-EFR3A is also found inside cells and somewhat on the lateral membrane. Bottom panels, ZO-1 (left panel) also partially colocalizes with GFP-RN-tre (US6NL (middle panel) and Merge (left panel); red, ZO-1; green, GFP). In this case, colocalization can be also seen in several intracellular vesicles (white arrows). Bar, 10 μm.
Similar articles
- Proteomic analysis of proteins surrounding occludin and claudin-4 reveals their proximity to signaling and trafficking networks.
Fredriksson K, Van Itallie CM, Aponte A, Gucek M, Tietgens AJ, Anderson JM. Fredriksson K, et al. PLoS One. 2015 Mar 19;10(3):e0117074. doi: 10.1371/journal.pone.0117074. eCollection 2015. PLoS One. 2015. PMID: 25789658 Free PMC article. - Connexin-occludin chimeras containing the ZO-binding domain of occludin localize at MDCK tight junctions and NRK cell contacts.
Mitic LL, Schneeberger EE, Fanning AS, Anderson JM. Mitic LL, et al. J Cell Biol. 1999 Aug 9;146(3):683-93. doi: 10.1083/jcb.146.3.683. J Cell Biol. 1999. PMID: 10444075 Free PMC article. - A complex of ZO-1 and the BAR-domain protein TOCA-1 regulates actin assembly at the tight junction.
Van Itallie CM, Tietgens AJ, Krystofiak E, Kachar B, Anderson JM. Van Itallie CM, et al. Mol Biol Cell. 2015 Aug 1;26(15):2769-87. doi: 10.1091/mbc.E15-04-0232. Epub 2015 Jun 10. Mol Biol Cell. 2015. PMID: 26063734 Free PMC article. - Epithelial junctions and Rho family GTPases: the zonular signalosome.
Citi S, Guerrera D, Spadaro D, Shah J. Citi S, et al. Small GTPases. 2014;5(4):1-15. doi: 10.4161/21541248.2014.973760. Small GTPases. 2014. PMID: 25483301 Free PMC article. Review. - Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival.
Kuo WT, Odenwald MA, Turner JR, Zuo L. Kuo WT, et al. Ann N Y Acad Sci. 2022 Aug;1514(1):21-33. doi: 10.1111/nyas.14798. Epub 2022 May 17. Ann N Y Acad Sci. 2022. PMID: 35580994 Free PMC article. Review.
Cited by
- BioID-based Identification of Skp Cullin F-box (SCF)β-TrCP1/2 E3 Ligase Substrates.
Coyaud E, Mis M, Laurent EM, Dunham WH, Couzens AL, Robitaille M, Gingras AC, Angers S, Raught B. Coyaud E, et al. Mol Cell Proteomics. 2015 Jul;14(7):1781-95. doi: 10.1074/mcp.M114.045658. Epub 2015 Apr 21. Mol Cell Proteomics. 2015. PMID: 25900982 Free PMC article. - Chronic high-dosage fish oil exacerbates gut-liver axis injury in alcoholic steatohepatitis in mice: the roles of endotoxin and IL-4 in Kupffer cell polarization imbalance.
Li XJ, Mu YM, Qin QF, Zeng ZX, Li YS, Zhang WK, Tang HB, Tian GH, Shang HC. Li XJ, et al. Toxicol Res (Camb). 2017 Jul 7;6(5):611-620. doi: 10.1039/c7tx00037e. eCollection 2017 Sep 1. Toxicol Res (Camb). 2017. PMID: 30090529 Free PMC article. - A Weak Link with Actin Organizes Tight Junctions to Control Epithelial Permeability.
Belardi B, Hamkins-Indik T, Harris AR, Kim J, Xu K, Fletcher DA. Belardi B, et al. Dev Cell. 2020 Sep 28;54(6):792-804.e7. doi: 10.1016/j.devcel.2020.07.022. Epub 2020 Aug 24. Dev Cell. 2020. PMID: 32841596 Free PMC article. - Membrane prewetting by condensates promotes tight-junction belt formation.
Pombo-García K, Adame-Arana O, Martin-Lemaitre C, Jülicher F, Honigmann A. Pombo-García K, et al. Nature. 2024 Aug;632(8025):647-655. doi: 10.1038/s41586-024-07726-0. Epub 2024 Aug 7. Nature. 2024. PMID: 39112699 Free PMC article. - Cingulin binds to the ZU5 domain of scaffolding protein ZO-1 to promote its extended conformation, stabilization, and tight junction accumulation.
Vasileva E, Spadaro D, Rouaud F, King JM, Flinois A, Shah J, Sluysmans S, Méan I, Jond L, Turner JR, Citi S. Vasileva E, et al. J Biol Chem. 2022 Apr;298(4):101797. doi: 10.1016/j.jbc.2022.101797. Epub 2022 Mar 5. J Biol Chem. 2022. PMID: 35259394 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials